Abstract
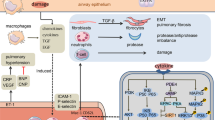
Therapeutic interventions in chronic obstructive pulmonary disease (COPD) shown to reduce exacerbations include smoking cessation, vaccination and appropriate pharmacological therapy. Long-acting bronchodilators are the cornerstone of COPD pharmacotherapy, whereas inhaled corticosteroids and mucolytics have shown benefit in subgroups of patients. Despite management with existing therapies, clinical trials confirm the persistent nature of exacerbations throughout the course of the disease. Roflumilast — a phosphodiesterase-4 (PDE4) inhibitor — received European Marketing Approval in 2010 and represents a new class of drug in the management of COPD. Through selective inhibition of the PDE4 enzyme, roflumilast prevents the breakdown of cyclic AMP, which plays an important role in regulating inflammatory cell activity. Early trials in patients with a forced expiratory volume in one second (FEV1) less than 50% predicted suggest that roflumilast offers sustained and significant improvement in lung function and a reduction in exacerbations compared with placebo, irrespective of concomitant bronchodilator therapy. Common adverse events include headache, diarrhoea and weight loss, with the majority occurring at the beginning of treatment, being transient and not leading to sequelae. Serious adverse events tended to be low across all studies. Roflumilast is currently licensed in Europe, and is indicated as maintenance treatment in severe COPD (i.e. in patients with post-bronchodilator FEV1 <50% predicted) associated with chronic bronchitis in adult patients with a history of frequent exacerbations as an add-on to bronchodilator treatment. Clear identification of patients eligible for roflumilast will require improved characterisation and phenotyping of patients in primary care, including lung function measurement, accurate health status classification, and recording of chronic cough and regular sputum production.
Similar content being viewed by others
Article PDF
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
David Price has consultant arrangements with Boehringer Ingelheim (BI), GlaxoSmithKline (GSK), Merck, Novartis and Teva. He or his research team have received grants and support for research in respiratory disease from the following organisations in the last 5 years: UK National Health Service, Aerocrine, AstraZeneca (AZ), BI, GSK, Merck, Novartis, Nycomed, Pfizer and Teva. He has spoken for: Boehringer Ingelheim, Chiesi, GlaxoSmithKline, Merck, Pfizer and Teva. He has shares in AKL Ltd which produces phytopharmaceuticals. He is the sole owner of Research in Real Life Ltd.
Alison Chisholm has no conflict of interest to declare.
Dermot Ryan has lectured on behalf of, received sponsorship from or provided consulatnacy services to: AZ, GSK, MSD, Schering Plough, Uriach Pharma, Chiesi, Nycomed, BI, Pfizer and Novartis Pharma. Alan Crockett has no conflict of interest to declare.
Rupert Jones has been paid to take part in advisory boards related to COPD for BI, GSK, Novartis, Nutricia, Pfizer, TEVA and in the last 3 years. RJ has spoken at scientific / educational meetings financed by Altana, AZ, BI, GSK, MSD, Pfizer, Tejin and Trinity Chiesi in the last 3 years. He is a consultant on the global emPOWER educational programme supported by Pfizer and Boehringer Ingelheim.
Rights and permissions
About this article
Cite this article
Price, D., Chisholm, A., Ryan, D. et al. The use of roflumilast in COPD: a primary care perspective. Prim Care Respir J 19, 342–351 (2010). https://doi.org/10.4104/pcrj.2010.00066
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.4104/pcrj.2010.00066
This article is cited by
-
Ecofriendly synthesis of 3-cyanopyridine derivatives by multi-component reaction catalyzed by animal bone meal
Environmental Chemistry Letters (2014)