Abstract
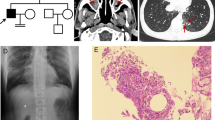
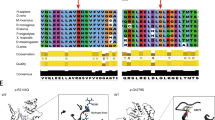
Primary ciliary dyskinesia (PCD) is a rare phenotypically and genetically heterogeneous disorder resulting from abnormal cilia ultrastructure and function. Few studies have reported the phenotype and genetic characteristics of PCD caused by mutations in DNAAF3. In this study, four PCD patients with DNAAF3 mutations underwent extensive clinical assessments, cilia ultrastructural and motion evaluations. All patients presented with situs inversus totalis, neonatal respiratory distress, and sinusitis; however, they did not have recurrent infections of the lower airways. The nasal nitric oxide level of these patients was markedly reduced. The respiratory cilia were found to be uniformly immotile, with their dynein arms defects. A total of 7 (5 novel) variants in DNAAF3 were identified and cosegregated in their families by Trio-based whole-exome sequencing. As the first report on DNAAF3 mutations in PCD patients in China, our study not only contributes to a deeper appreciation of the phenotypic characteristics of patients with DNAAF3 mutations but also expands the spectrum of DNAAF3 mutations and may contribute to the genetic diagnosis of and counseling for PCD.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Reula A, Lucas JS, Morenogaldó A, Romero T, Milara X. New insights in primary ciliary dyskinesia. Expert Opin Orphan Drugs. 2017;5:537–48.
Knowles MR, Daniels LA, Davis SD, Zariwala MA, Leigh MW. Primary ciliary dyskinesia. Recent advances in diagnostics, genetics, and characterization of clinical disease. Am J Respir Crit Care Med. 2013;188:913–22.
Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–93.
Bonnefoy S, Watson CM, Kernohan KD, Lemos M, Hutchinson S, Poulter JA, et al. Biallelic mutations in LRRC56, encoding a protein associated with intraflagellar transport, cause mucociliary clearance and laterality defects. Am J Hum Genet. 2018;103:727–39.
Loges NT, Antony D, Maver A, Deardorff MA, Güleç EY, Gezdirici A, et al. Recessive DNAH9 loss-of-function mutations cause laterality defects and subtle respiratory ciliary-beating defects. Am J Hum Genet. 2018;103:995–1008.
Fassad MR, Shoemark A, Borgne PI, Koll F, Patel M, et al. C11orf70 mutations disrupting the intraflagellar transport-dependent assembly of multiple axonemal dyneins cause primary ciliary dyskinesia. Am J Hum Genet. 2018;5:956–72.
Knowles MR, Ostrowski LE, Leigh MW, Sears PR, Davis SD, Wolf WE, et al. Mutations in RSPH1 cause primary ciliary dyskinesia with a unique clinical and ciliary phenotype. Am J Respir Crit Care Med. 2014;189:707–17.
Davis SD, Ferkol TW, Rosenfeld M, Lee HS, Dell SD, Sagel SD et al. Clinical features of childhood primary ciliary dyskinesia by genotype and ultrastructural phenotype. Am J Respir Crit Care Med. 2015;191:316–24.
Mitchison HM, Schmidts M, Loges NT, Freshour J, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44:381–9.
Lucas JS, Barbato A, Collins SA, Goutaki M, Behan L, Caudri D, et al. European Respiratory Society guidelines for the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2017;49:1601090.
American Thoracic Society, European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide. Am J Respir Crit Care Med. 2005;171:912–30.
Beydon N, Chambellan A, Alberti C, de Blic J, Clement A, Escudier E, et al. Technical and practical issues for tidal breathing measurements of nasal nitric oxide in children. Pediatr Pulmonol. 2015;50:1374–82.
DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet. 2011;43:491–8.
Yang L, Kong Y, Dong X, Hu L, Lin Y, Chen X, et al. Clinical and genetic spectrum of a large cohort of children with epilepsy in China. Genet Med. 2018. https://www.ncbi.nlm.nih.gov/.
Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med. 2015;17:405–24.
Fromer M, Pocklington AJ, Kavanagh DH, Williams HJ, Dwyer S, Gormley P, et al. De novo mutations in schizophrenia implicate synaptic networks. Nature. 2014;506:179–84.
Leigh MW, Hazucha MJ, Chawla KK, Baker BR, Shapiro AJ, Brown DE, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia. Ann Am Thorac Soc. 2013;10:574–81.
Olbrich H, Cremers C, Loges NT, Werner C, Nielsen KG, Marthin JK, et al. Loss-of-function GAS8 mutations cause primary ciliary dyskinesia and disrupt the nexin–dynein regulatory complex. Am J Hum Genet. 2015;97:546–54.
Shoemark A, Moya E, Hirst RA, Patel MP, Robson EA, Hayward J, et al. High prevalence of CCDC103 p.His154Pro mutation causing primary ciliary dyskinesia disrupts protein oligomerisation and is associated with normal diagnostic investigations. Thorax. 2018;73:157–66.
You S, Zhang J, Bai Y, Ji L, Wang H. Normal values of nasal NO and exhaled NO in young Chinese people aged 9–22 years. World J Otorhinolaryngol Head Neck Surg. 2016;2:22–7.
Davis SD, Rosenfeld M, Lee HS, Ferkol TW, Sage SD, et al. Primary ciliary dyskinesia: longitudinal study of lung disease by ultrastructure defect and genotype. Am J Respir Crit Care Med. 2019;199:190–8.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (81471481), the Development Fund for Shanghai Talents (201450), and the Open Research Project of the Shanghai Key Laboratory of Birth Defects (16DZKF1012).
Author information
Authors and Affiliations
Contributions
Zhuoyao Guo and Weicheng Chen performed the research, analyzed and interpreted the data, and drafted the manuscript. Jianfeng Huang designed the study and analyzed the data. Libo Wang and Liling Qian conceived and designed the study, revised the manuscript, and provided final approval of the manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guo, Z., Chen, W., Huang, J. et al. Clinical and genetic analysis of patients with primary ciliary dyskinesia caused by novel DNAAF3 mutations. J Hum Genet 64, 711–719 (2019). https://doi.org/10.1038/s10038-019-0609-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s10038-019-0609-1
This article is cited by
-
Single cell RNA analysis of the left–right organizer transcriptome reveals potential novel heterotaxy genes
Scientific Reports (2023)
-
Key gene network related to primary ciliary dyskinesia in hippocampus of patients with Alzheimer’s disease revealed by weighted gene co-expression network analysis
BMC Neurology (2022)
-
Biallelic loss of function NEK3 mutations deacetylate α-tubulin and downregulate NUP205 that predispose individuals to cilia-related abnormal cardiac left–right patterning
Cell Death & Disease (2020)