Abstract
Background
High-fat diet (HFD) induces negative effects on the activity of interscapular brown adipose tissue (iBAT) and systemic energy metabolism. Irisin, a small hormonal agent known to modulate metabolism has been used for intervening HFD-induced obesity. However, its mechanism of action on iBAT function remains to be fully elucidated. This study sought to investigate whether irisin intervention could restore the thermogenic function of iBAT in mice with HFD-induced obesity, thereby regulating systemic metabolism.
Methods
Magnetic resonance imaging (MRI) and positron emission tomography/computed tomography (PET/CT) were used to monitor changes of thermogenic capacity of iBAT and systemic metabolism in mice with HFD-induced obesity and iBAT deficiency during 2-week or 4-week irisin intervention. Pathological and molecular biology analyses were performed on tissue and blood samples.
Results
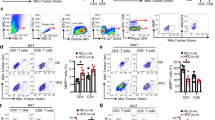
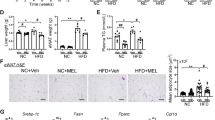
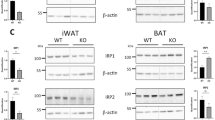
Prolonged HFD feeding in mice induced obesity and impaired the thermogenic capacity of iBAT. MRI results showed that irisin intervention for 4-week reduced lipid content in iBAT, increased uncoupling protein 1 (UCP 1) expression and enhanced glucose analogue uptake capacity. These improvements of functions in iBAT activity were accompanied by an improvement in systemic metabolism. The positive effects of irisin appears to be dependent on the length of intervention time. When iBAT was removed, the beneficial effects of irisin were partially suppressed, suggesting that irisin regulates metabolism through the restoration of the thermogenic function of iBAT.
Conclusions
HFD results in reduced thermogenic capacity of iBAT, while irisin intervention can effectively restore iBAT function, leading to improvement in overall glucose and lipid metabolism.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Becher T, Palanisamy S, Kramer DJ, Eljalby M, Marx SJ, Wibmer AG, et al. Brown adipose tissue is associated with cardiometabolic health. Nat Med. 2021;27:58–65.
Liu X, Wang S, You Y, Meng M, Zheng Z, Dong M, et al. Brown adipose tissue transplantation reverses obesity in Ob/Ob mice. Endocrinology. 2015;156:2461–9.
Scheele C, Wolfrum C. Brown adipose crosstalk in tissue plasticity and human metabolism. Endocr Rev. 2020;41:53–65.
Sveidahl Johansen O, Ma T, Hansen JB, Markussen LK, Schreiber R, Reverte-Salisa L, et al. Lipolysis drives expression of the constitutively active receptor GPR3 to induce adipose thermogenesis. Cell. 2021;184:3502–18.e33.
Jacobsson A, Stadler U, Glotzer MA, Kozak LP. Mitochondrial uncoupling protein from mouse brown fat. Molecular cloning, genetic mapping, and mRNA expression. J Biol Chem. 1985;260:16250–4.
Porter C, Herndon DN, Chondronikola M, Chao T, Annamalai P, Bhattarai N, et al. Human and mouse brown adipose tissue mitochondria have comparable UCP1 function. Cell Metab. 2016;24:246–55.
Rangel-Azevedo C, Santana-Oliveira DA, Miranda CS, Martins FF, Mandarim-de-Lacerda CA, Souza-Mello V. Progressive brown adipocyte dysfunction: Whitening and impaired nonshivering thermogenesis as long-term obesity complications. J Nutr Biochem. 2022;105:109002.
Gross B, Pawlak M, Lefebvre P, Staels B. PPARs in obesity-induced T2DM, dyslipidaemia and NAFLD. Nat Rev Endocrinol. 2017;13:36–49.
Turpin SM, Nicholls HT, Willmes DM, Mourier A, Brodesser S, Wunderlich CM, et al. Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab. 2014;20:678–86.
Rozec B, Gauthier C. beta3-adrenoceptors in the cardiovascular system: putative roles in human pathologies. Pharmacol Ther. 2006;111:652–73.
Michel LYM, Farah C, Balligand JL. The Beta3 adrenergic receptor in healthy and pathological cardiovascular tissues. Cells. 2020;9:2584.
Cypess AM, Weiner LS, Roberts-Toler C, Franquet Elía E, Kessler SH, Kahn PA, et al. Activation of human brown adipose tissue by a β3-adrenergic receptor agonist. Cell Metab. 2015;21:33–8.
Boström P, Wu J, Jedrychowski M, Korde A, Ye L, Lo J, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–8.
Zhang Y, Xie C, Wang H, Foss RM, Clare M, George EV, et al. Irisin exerts dual effects on browning and adipogenesis of human white adipocytes. Am J Physiol Endocrinol Metab. 2016;311:E530–41.
Guo M, Yao J, Li J, Zhang J, Wang D, Zuo H, et al. Irisin ameliorates age-associated sarcopenia and metabolic dysfunction. J Cachexia Sarcopenia Muscle. 2023;14:391–405.
Zhang Y, Li R, Meng Y, Li S, Donelan W, Zhao Y, et al. Irisin stimulates browning of white adipocytes through mitogen-activated protein kinase p38 MAP kinase and ERK MAP kinase signaling. Diabetes. 2014;63:514–25.
Zhang HJ, Zhang XF, Ma ZM, Pan LL, Chen Z, Han HW, et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J Hepatol. 2013;59:557–62.
Shimizu I, Aprahamian T, Kikuchi R, Shimizu A, Papanicolaou KN, MacLauchlan S, et al. Vascular rarefaction mediates whitening of brown fat in obesity. J Clin Invest. 2014;124:2099–112.
Peng XG, Ju S, Fang F, Wang Y, Fang K, Cui X, et al. Comparison of brown and white adipose tissue fat fractions in ob, seipin, and Fsp27 gene knockout mice by chemical shift-selective imaging and (1)H-MR spectroscopy. Am J Physiol Endocrinol Metab. 2013;304:E160–7.
Chen Y, Ding J, Zhao Y, Ju S, Mao H, Peng XG. Irisin induces white adipose tissue browning in mice as assessed by magnetic resonance imaging. Exp Biol Med (Maywood). 2021;246:1597–606.
Zhao Y, Dai J, Jiang Y, Wu H, Cui Y, Li X, et al. Reducing white adipose tissue browning using p38α MAPK inhibitors ameliorates cancer-associated cachexia as assessed by magnetic resonance imaging. Nutrients. 2022;14:3013.
Kong X, Yao T, Zhou P, Kazak L, Tenen D, Lyubetskaya A, et al. Brown adipose tissue controls skeletal muscle function via the secretion of myostatin. Cell Metab. 2018;28:631–43.e3.
Lunati E, Marzola P, Nicolato E, Fedrigo M, Villa M, Sbarbati A. In vivo quantitative lipidic map of brown adipose tissue by chemical shift imaging at 4.7 Tesla. J Lipid Res. 1999;40:1395–400.
Seki T, Yang Y, Sun X, Lim S, Xie S, Guo Z, et al. Brown-fat-mediated tumour suppression by cold-altered global metabolism. Nature. 2022;608:421–8.
Mirbolooki MR, Constantinescu CC, Pan ML, Mukherjee J. Quantitative assessment of brown adipose tissue metabolic activity and volume using 18F-FDG PET/CT and β3-adrenergic receptor activation. EJNMMI Res. 2011;1:30.
Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. 2017;114:8649–54.
Song A, Dai W, Jang MJ, Medrano L, Li Z, Zhao H, et al. Low- and high-thermogenic brown adipocyte subpopulations coexist in murine adipose tissue. J Clin Invest. 2020;130:247–57.
Crandall JP, Fraum TJ, Wahl RL. Brown adipose tissue: a protective mechanism against “preprediabetes”? J Nucl Med. 2022;63:1433–40.
Cobb T, Hwang I, Soukar M, Namkoong S, Cho US, Safdar M, et al. Iditarod, a Drosophila homolog of the Irisin precursor FNDC5, is critical for exercise performance and cardiac autophagy. Proc Natl Acad Sci USA. 2023;120:e2220556120.
Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, Idelson C, et al. Irisin and FGF21 are cold-induced endocrine activators of brown fat function in humans. Cell Metab. 2014;19:302–9.
Martinez-Lopez N, Garcia-Macia M, Sahu S, Athonvarangkul D, Liebling E, Merlo P, et al. Autophagy in the CNS and periphery coordinate lipophagy and lipolysis in the brown adipose tissue and liver. Cell Metab. 2016;23:113–27.
Lee J, Ellis JM, Wolfgang MJ. Adipose fatty acid oxidation is required for thermogenesis and potentiates oxidative stress-induced inflammation. Cell Rep. 2015;10:266–79.
Wang J, Fang N, Xiong J, Du Y, Cao Y, Ji WK. An ESCRT-dependent step in fatty acid transfer from lipid droplets to mitochondria through VPS13D-TSG101 interactions. Nat Commun. 2021;12:1252.
Sponton CH, de Lima-Junior JC, Leiria LO. What puts the heat on thermogenic fat: metabolism of fuel substrates. Trends Endocrinol Metab. 2022;33:587–99.
Leiria LO, Wang CH, Lynes MD, Yang K, Shamsi F, Sato M, et al. 12-Lipoxygenase Regulates Cold Adaptation and Glucose Metabolism by Producing the Omega-3 Lipid 12-HEPE from Brown Fat. Cell Metab. 2019;30:768–83.e7.
Duta-Mare M, Sachdev V, Leopold C, Kolb D, Vujic N, Korbelius M, et al. Lysosomal acid lipase regulates fatty acid channeling in brown adipose tissue to maintain thermogenesis. Biochim Biophys Acta Mol Cell Biol Lipids. 2018;1863:467–78.
Cui L, Mirza AH, Zhang S, Liang B, Liu P. Lipid droplets and mitochondria are anchored during brown adipocyte differentiation. Protein Cell. 2019;10:921–6.
Yu J, Zhang S, Cui L, Wang W, Na H, Zhu X, et al. Lipid droplet remodeling and interaction with mitochondria in mouse brown adipose tissue during cold treatment. Biochim Biophys Acta. 2015;1853:918–28.
Franz D, Karampinos DC, Rummeny EJ, Souvatzoglou M, Beer AJ, Nekolla SG, et al. Discrimination Between Brown and White Adipose Tissue Using a 2-Point Dixon Water-Fat Separation Method in Simultaneous PET/MRI. J Nucl Med. 2015;56:1742–7.
Hamilton G, Smith DL, Bydder M, Nayak KS, Hu HH. MR properties of brown and white adipose tissues. J Magn Reson Imaging. 2011;34:468–73.
Ouwerkerk R, Hamimi A, Matta J, Abd-Elmoniem KZ, Eary JF, Abdul Sater Z, et al. Proton MR Spectroscopy Measurements of White and Brown Adipose Tissue in Healthy Humans: Relaxation Parameters and Unsaturated Fatty Acids. Radiology. 2021;299:396–406.
Lundström E, Strand R, Forslund A, Bergsten P, Weghuber D, Ahlström H, et al. Automated segmentation of human cervical-supraclavicular adipose tissue in magnetic resonance images. Sci Rep. 2017;7:3064.
Chouchani ET, Kazak L, Spiegelman BM. New advances in adaptive thermogenesis: UCP1 and Beyond. Cell Metab. 2019;29:27–37.
Ikeda K, Kang Q, Yoneshiro T, Camporez JP, Maki H, Homma M, et al. UCP1-independent signaling involving SERCA2b-mediated calcium cycling regulates beige fat thermogenesis and systemic glucose homeostasis. Nat Med. 2017;23:1454–65.
Kazak L, Chouchani ET, Jedrychowski MP, Erickson BK, Shinoda K, Cohen P, et al. A creatine-driven substrate cycle enhances energy expenditure and thermogenesis in beige fat. Cell. 2015;163:643–55.
Wang T, Sharma AK, Wu C, Maushart CI, Ghosh A, Yang W, et al. Single-nucleus transcriptomics identifies separate classes of UCP1 and futile cycle adipocytes. Cell Metab. 2024;36:2130–45.e7.
Acknowledgements
This work is funded by National Natural Science Foundation of China (81871412 and 82272064), Jiangsu Provincial Science and Technique Program (BK20221461), Postgraduate Research & Practice Innovation Program of Jiangsu Province (KYCX21_0159, KYCX22_0297, and KYCX23_0323).
Author information
Authors and Affiliations
Contributions
The contributions of all authors are presented as follows. Jingyue Dai: research design and conceptualization, background research, practical performance, data analysis, writing - original draft. Yufei Zhao, Xingzhe Tang, Rui Sun: validation, practical performance. Yue Chen: research design and conceptualization, background research. Yang Jiang, Ying Cui: data collection and analysis. Hui Mao: experimental design, writing - review & editing. Xin-Gui Peng: research design and conceptualization, supervision, project administration, funding acquisition, writing - review & editing. All authors revised and edited the manuscript. The authors extend thanks to Dr. Xiaoxuan Xu, Prof. Yanli An and Prof. Fengchao Zang for their invaluable assistance in methodological support.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Ethics Approval and Consent to Participate
All animal experiments were performed in accordance with the relevant guidelines and regulations approved by the Institutional Animal Care and Use Committee of the Medical School of Southeast University (approval ID: 20210601020).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Dai, J., Zhao, Y., Chen, Y. et al. Irisin reverses high-fat diet-induced metabolic dysfunction via activation of brown adipose tissue in mice. Int J Obes 49, 1066–1075 (2025). https://doi.org/10.1038/s41366-025-01739-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41366-025-01739-z