Abstract
Background
Administering darbepoetin (Darbe) to preterm infants stimulates erythropoiesis, increases red blood cell (RBC) mass, and reduces RBC transfusions. We typically administer 10 µg/kg weekly until 34 weeks corrected gestation; however, we are uncertain whether this dose could be given every other week (biweekly) with equal efficacy.
Methods
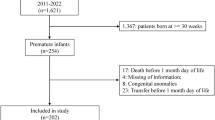
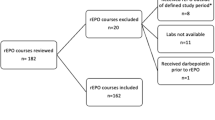
Infants ≤32 weeks were randomized to receive Darbe 10 µg/kg/dose weekly or biweekly for six weeks, tracking complete blood counts, absolute reticulocyte counts (ARC), iron status (RET-He), and RBC transfusions.
Results
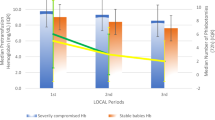
We enrolled 71 infants (1027 ± 369 grams, 27.3 ± 2.8 weeks). During the study period, the weekly-dosed group had a higher adjusted mean ARC (48,000/μL higher, 95% C.I. 9700–87,000/μL; p = 0.019). However, RET-He, Hgb, and transfusion-free survival were not different between groups (p = 0.071, p = 0.244, and p = 0.762).
Conclusions
Weekly Darbe results in a higher six-week ARC than biweekly dosing. However, given similar clinical outcomes, perhaps biweekly dosing may be a cost-effective alternative.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
Inquiries regarding sharing de-identified data sets and computer code can by made to the corresponding author. Deidentified data will be shared on request to the corresponding author.
Code availability
Inquiries regarding sharing de-identified data sets and computer code can by made to the corresponding author.
References
Khullar D, Muchhala SS, Abhishek T. Advancing anemia management in chronic kidney disease: Assessing the superiority of darbepoetin alfa over erythropoietin alpha. Cureus. 2024;16:e51613.
Mazahir R, Anand K, Pruthi PK. Comparison of darbepoetin alpha and recombinant human erythropoietin for treatment of anemia in pediatric chronic kidney disease: a non-inferiority trial from India. Eur J Pediatr. 2023;182:101–9.
Ohls RK, Dai A. Long-acting erythropoietin: clinical studies and potential uses in neonates. Clin Perinatol. 2004;31:77–89.
Ohls RK, Christensen RD, Kamath-Rayne BD, Rosenberg A, Wiedmeier SE, Roohi M, et al. A randomized, masked, placebo-controlled study of darbepoetin alfa in preterm infants. Pediatrics. 2013;132:e119–27.
Ree IMC, de Haas M, van Geloven N, Juul SE, de Winter D, Verweij EJT, et al. Darbepoetin alfa to reduce transfusion episodes in infants with haemolytic disease of the fetus and newborn who are treated with intrauterine transfusions in the Netherlands: an open-label, single-centre, phase 2, randomised, controlled trial. Lancet Haematol. 2023;10:e976–84.
Ohls RK, Bahr TM, Peterson TG, Christensen RD. A practical guide to reducing/eliminating red blood cell transfusions in the neonatal intensive care unit. Semin Fetal Neonatal Med. 2024:101545. https://doi.org/10.1016/j.siny.2024.101545.
Warwood TL, Ohls RK, Wiedmeier SE, Lambert DK, Jones C, Scoffield SH, et al. Single dose darbepoetin administration to anemic preterm neonates. J Perinatol. 2005;25:725–30.
An G, Ohls RK, Christensen RD, Widness JA, Mock DM, Veng-Pedersen P. Population pharmacokinetics of Darbepoetin in infants following single intravenous and subcutaneous dosing. J Pharm Sci. 2017;106:1644–9.
Macdougall IC, Gray SJ, Elston O, Breen C, Jenkins B, Browne J, et al. Pharmacokinetics of novel erythropoiesis stimulating protein compared with epoetin alfa in dialysis patients. J Am Soc Nephrol. 1999;10:2392–5.
Ohls RK, Kamath-Rayne BD, Christensen RD, Wiedmeier SE, Rosenberg A, Fuller J, et al. Cognitive outcomes of preterm infants randomized to darbepoetin, erythropoietin, or placebo. Pediatrics. 2014;133:1023–30.
Bahr TM, Knudsen MC, Lozano-Chinga M, Agarwal AM, Meznarich JA, Ohls RK, et al. Infantile Pyknocytosis: end-tidal CO, %Micro-R measurements, next-generation sequencing, and transfusion avoidance with darbepoetin. Biomed Hub. 2020;5:227–34.
Ohlsson A, Aher SM. Early erythropoiesis-stimulating agents in preterm or low birth weight infants. Cochrane Database Syst Rev. 2020;2:CD004863.
Bahr TM, Ohls RK, Christensen RD. Treating preterm infants with erythropoietin does not increase the risk of retinopathy of prematurity. J Perinatol. 2025;45:292–3.
Ohls RK, Schibler KR, Lowe JR, Tan S, Beauman S. Darbepoetin trial to improve red cell mass and neuroprotection in preterm infants. Oral Platform. Annual meeting. Pediatric Academic Society; 2023. https://2023.pas-meeting.org/.
Maier RF, Sonntag J, Walka MM, Liu G, Metze BC, Obladen M. Changing practices of red blood cell transfusions in infants with birth weights less than 1000 g. J Pediatr. 2000;136:220–4.
Wu DW, Friedman MT, Lombardi DP, Hwang R, Sender J, Cobaj V, et al. Impact of patient blood management on red blood cell utilization in an urban community teaching hospital: A seven-year retrospective study. Life. 2024;14:232.
Bahr TM, Snow GL, Christensen TR, Davenport P, Henry E, Tweddell SM, et al. Can red blood cell and platelet transfusions have a pathogenic role in bronchopulmonary dysplasia? J Pediatr. 2024;265:113836.
Bahr TM, Ohls RK, Henry E, Davenport P, Ilstrup SJ, Kelley WE, et al. The number of blood transfusions received and the incidence and severity of chronic lung disease among NICU patients born >31 weeks gestation. J Perinatol. 2024. https://doi.org/10.1038/s41372-024-02135-7.
Teofili L, Papacci P, Dani C, Cresi F, Remaschi G, Pellegrino C, et al. Cord blood transfusions in extremely low gestational age neonates to reduce severe retinopathy of prematurity: results of a prespecified interim analysis of the randomized BORN trial. Ital J Pediatr. 2024;50:142.
Kirpalani H, Bell EF, Hintz SR, Tan S, Schmidt B, Chaudhary AS, et al. Eunice Kennedy Shriver NICHD Neonatal Research Network. Higher or Lower Hemoglobin Transfusion Thresholds for Preterm Infants. N Engl J Med. 2020;383:2639–51.
Acknowledgements
The authors thank the parents of the study subjects for their willingness to allow their infants to participate, and the staff and coordinators at the Newborn Intensive Care Units at Utah Valley Hospital and Intermountain Medical Center.
Author information
Authors and Affiliations
Contributions
TMB, RDC, and RKO; conception and design, assembly of data, data analysis, manuscript writing, final approval of the manuscript. KAW-L; overseeing nursing participation, final approval of the manuscript. DMS; conception and design, overseeing pharmacy participation, final approval of the manuscript. JJT, EG; conception and design and final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bahr, T.M., Christensen, R.D., Weaver-Lewis, K.A. et al. A prospective randomized pilot trial comparing weekly vs. biweekly Darbepoetin administration to preterm infants. J Perinatol (2025). https://doi.org/10.1038/s41372-025-02247-8
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41372-025-02247-8