Abstract
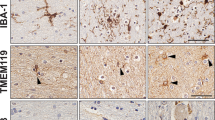
It is known that infections with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) cause coronavirus disease 2019 (COVID-19). It is widely reported that Alzheimer’s disease (AD) is associated with the highest risk of COVID-19 infection, hospitalization and mortality. However, it remains largely unclear about the link between AD and COVID-19. ACE2 is an entry receptor for SARS-CoV-2. We consider that there may be a link between AD and COVID-19 through the expression of ACE2. Here, we summarize recent findings about the ACE2 expression especially in AD and COVID-19, and shows that (1) ACE2 shows mRNA and protein expression in human brain tissues, especially in neurons and non-neuron cells; (2) low ACE2 mRNA and protein expression are sufficient for SARS-CoV-2 entry into the human brain through the neural route (olfactory and/or vagal) and the hematogenous route; (3) SARS-CoV-2 RNA and protein were detected in brains of COVID-19 patients; (4) SARS-CoV-2 infects and replicates in human brain dependent on ACE2; (5) SARS-CoV-2 viral RNA load shows a positive association with ACE2 mRNA levels and COVID-19 severity; (6) ACE2 shows increased expression in AD compared with controls in human brain; (7) ACE2 shows increased expression in COVID-19 compared with controls in human brain; (8) ACE2 expression levels affect COVID-19 outcomes. Together, ACE2 shows significantly increased mRNA and protein expression in AD compared with controls in human brain. Consequently, the increased expression of ACE2 would facilitate infection with SARS-CoV-2, and play a role in the context of COVID-19. These findings suggest that the expression of ACE2 may partly explain the link of AD with COVID-19 infection, hospitalization and mortality.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
All relevant data are within the paper. The authors confirm that all data underlying the findings are either fully available without restriction through consortia websites, or may be made available from consortia upon request.
References
Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–20.
Nishiga M, Wang DW, Han Y, Lewis DB, Wu JC. COVID-19 and cardiovascular disease: from basic mechanisms to clinical perspectives. Nat Rev Cardiol. 2020;17:543–58.
Deigendesch N, Sironi L, Kutza M, Wischnewski S, Fuchs V, Hench J, et al. Correlates of critical illness-related encephalopathy predominate postmortem COVID-19 neuropathology. Acta Neuropathol. 2020;140:583–6.
Atkins JL, Masoli JAH, Delgado J, Pilling LC, Kuo CL, Kuchel GA, et al. Preexisting comorbidities predicting COVID-19 and mortality in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2224–30.
Kuo CL, Pilling LC, Atkins JL, Masoli JAH, Delgado J, Kuchel GA, et al. APOE e4 genotype predicts severe COVID-19 in the UK Biobank Community Cohort. J Gerontol A Biol Sci Med Sci. 2020;75:2231–2.
Mok VCT, Pendlebury S, Wong A, Alladi S, Au L, Bath PM, et al. Tackling challenges in care of Alzheimer’s disease and other dementias amid the COVID-19 pandemic, now and in the future. Alzheimers Dement. 2020;16:1571–81.
Yu Y, Travaglio M, Popovic R, Leal NS, Martins LM. Alzheimer’s and Parkinson’s diseases predict different COVID-19 outcomes: A UK Biobank Study. Geriatrics. 2021;6:10.
Tahira AC, Verjovski-Almeida S, Ferreira ST. Dementia is an age-independent risk factor for severity and death in COVID-19 inpatients. Alzheimers Dement. 2021;17:1818–31.
Wang Q, Davis PB, Gurney ME, Xu R. COVID-19 and dementia: analyses of risk, disparity, and outcomes from electronic health records in the US. Alzheimers Dement. 2021;17:1297–306.
Mangal R, Ding Y. Mini review: prospective therapeutic targets of Alzheimer’s disease. Brain Circ. 2022;8:1–5.
Thakkar N, Martis PB, Kutikuppala LVS, Kuchana SK, Mohapatra RK. Lecanemab: a hope in the management of Alzheimer’s disease. Brain Circ. 2023;9:194–5.
Docherty AB, Harrison EM, Green CA, Hardwick HE, Pius R, Norman L, et al. Features of 20 133 UK patients in hospital with covid-19 using the ISARIC WHO clinical characterisation protocol: prospective observational cohort study. BMJ. 2020;369:m1985.
Hoffmann M, Kleine-Weber H, Schroeder S, Kruger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–80.
Wang Y, Luo W, Huang L, Xiao J, Li F, Qin S, et al. A comprehensive investigation of the mRNA and protein level of ACE2, the putative receptor of SARS-CoV-2, in human tissues and blood cells. Int J Med Sci. 2020;17:1522–31.
Chen R, Wang K, Yu J, Howard D, French L, Chen Z, et al. The spatial and cell-type distribution of SARS-CoV-2 receptor ACE2 in the human and mouse brains. Front Neurol. 2020;11:573095.
Hikmet F, Mear L, Edvinsson A, Micke P, Uhlen M, Lindskog C. The protein expression profile of ACE2 in human tissues. Mol Syst Biol. 2020;16:e9610.
Lukiw WJ, Pogue A, Hill JM. SARS-CoV-2 infectivity and neurological targets in the brain. Cell Mol Neurobiol. 2022;42:217–24.
Li MY, Li L, Zhang Y, Wang XS. Expression of the SARS-CoV-2 cell receptor gene ACE2 in a wide variety of human tissues. Infect Dis Poverty. 2020;9:45.
Battle A, Brown CD, Engelhardt BE, Montgomery SB. Genetic effects on gene expression across human tissues. Nature. 2017;550:204–13.
Katz J, Yue S, Xue W, Gao H. Increased odds ratio for erectile dysfunction in COVID-19 patients. J Endocrinol Invest. 2022;45:859–64.
Xu E, Xie Y, Al-Aly Z. Long-term gastrointestinal outcomes of COVID-19. Nat Commun. 2023;14:983.
Lee K, Park J, Lee J, Lee M, Kim HJ, Son Y, et al. Long-term gastrointestinal and hepatobiliary outcomes of COVID-19: a multinational population-based cohort study from South Korea, Japan, and the UK. Clin Mol Hepatol. 2024;30:943–58.
Lindskog C, Mear L, Virhammar J, Fallmar D, Kumlien E, Hesselager G, et al. Protein expression profile of ACE2 in the normal and COVID-19-affected human brain. J Proteome Res. 2022;21:2137–45.
Cui H, Su S, Cao Y, Ma C, Qiu W. The altered anatomical distribution of ACE2 in the brain with Alzheimer’s disease pathology. Front Cell Dev Biol. 2021;9:684874.
Fodoulian L, Tuberosa J, Rossier D, Boillat M, Kan C, Pauli V, et al. SARS-CoV-2 receptors and entry genes are expressed in the human olfactory neuroepithelium and brain. iScience. 2020;23:101839.
Matschke J, Lutgehetmann M, Hagel C, Sperhake JP, Schroder AS, Edler C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020;19:919–29.
Song E, Zhang C, Israelow B, Lu-Culligan A, Prado AV, Skriabine S, et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J Exp Med. 2021;218:e20202135.
Xu J, Lazartigues E. Expression of ACE2 in human neurons supports the neuro-invasive potential of COVID-19 virus. Cell Mol Neurobiol. 2022;42:305–9.
Mukerjee S, Gao H, Xu J, Sato R, Zsombok A, Lazartigues E. ACE2 and ADAM17 interaction regulates the activity of presympathetic neurons. Hypertension. 2019;74:1181–91.
Feng Y, Xia H, Cai Y, Halabi CM, Becker LK, Santos RA, et al. Brain-selective overexpression of human Angiotensin-converting enzyme type 2 attenuates neurogenic hypertension. Circ Res. 2010;106:373–82.
Sriramula S, Xia H, Xu P, Lazartigues E. Brain-targeted angiotensin-converting enzyme 2 overexpression attenuates neurogenic hypertension by inhibiting cyclooxygenase-mediated inflammation. Hypertension. 2015;65:577–86.
Chen J, Zhao Y, Chen S, Wang J, Xiao X, Ma X, et al. Neuronal over-expression of ACE2 protects brain from ischemia-induced damage. Neuropharmacology. 2014;79:550–8.
Zhang X, Zhang Y, Zhang L, Qin C. Overexpression of ACE2 ameliorates Abeta-induced blood-brain barrier damage and angiogenesis by inhibiting NF-kappaB/VEGF/VEGFR2 pathway. Animal Model Exp Med. 2023;6:237–44.
Reynolds JL, Mahajan SD. SARS-COV2 alters blood brain barrier integrity contributing to neuro-inflammation. J Neuroimmune Pharmacol. 2021;16:4–6.
Meinhardt J, Radke J, Dittmayer C, Franz J, Thomas C, Mothes R, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021;24:168–75.
Bulfamante G, Bocci T, Falleni M, Campiglio L, Coppola S, Tosi D, et al. Brainstem neuropathology in two cases of COVID-19: SARS-CoV-2 trafficking between brain and lung. J Neurol. 2021;268:4486–91.
Wenzel J, Lampe J, Muller-Fielitz H, Schuster R, Zille M, Muller K, et al. The SARS-CoV-2 main protease M(pro) causes microvascular brain pathology by cleaving NEMO in brain endothelial cells. Nat Neurosci. 2021;24:1522–33.
Stein SR, Ramelli SC, Grazioli A, Chung JY, Singh M, Yinda CK, et al. SARS-CoV-2 infection and persistence in the human body and brain at autopsy. Nature. 2022;612:758–63.
Marshall M. COVID and the brain: researchers zero in on how damage occurs. Nature. 2021;595:484–5.
Wang L, Sievert D, Clark AE, Lee S, Federman H, Gastfriend BD, et al. A human three-dimensional neural-perivascular ‘assembloid’ promotes astrocytic development and enables modeling of SARS-CoV-2 neuropathology. Nat Med. 2021;27:1600–6.
Viszlayova D, Sojka M, Dobrodenkova S, Szabo S, Bilec O, Turzova M, et al. SARS-CoV-2 RNA in the cerebrospinal fluid of a patient with long COVID. Ther Adv Infect Dis. 2021;8:20499361211048572.
Luis MB, Liguori NF, Lopez PA, Alonso R. SARS-CoV-2 RNA detection in cerebrospinal fluid: presentation of two cases and review of literature. Brain Behav Immun Health. 2021;15:100282.
Virhammar J, Kumlien E, Fallmar D, Frithiof R, Jackmann S, Skold MK, et al. Acute necrotizing encephalopathy with SARS-CoV-2 RNA confirmed in cerebrospinal fluid. Neurology. 2020;95:445–9.
Xiang P, Xu X, Lu X, Gao L, Wang H, Li Z, et al. Case report: identification of SARS-CoV-2 in cerebrospinal fluid by ultrahigh-depth sequencing in a patient with coronavirus disease 2019 and neurological dysfunction. Front Med. 2021;8:629828.
Zhang L, Zhou L, Bao L, Liu J, Zhu H, Lv Q, et al. SARS-CoV-2 crosses the blood-brain barrier accompanied with basement membrane disruption without tight junctions alteration. Signal Transduct Target Ther. 2021;6:337.
Rhea EM, Logsdon AF, Hansen KM, Williams LM, Reed MJ, Baumann KK, et al. The S1 protein of SARS-CoV-2 crosses the blood-brain barrier in mice. Nat Neurosci. 2021;24:368–78.
Lempriere S. SARS-CoV-2 detected in olfactory neurons. Nat Rev Neurol. 2021;17:63.
Jiao L, Yang Y, Yu W, Zhao Y, Long H, Gao J, et al. The olfactory route is a potential way for SARS-CoV-2 to invade the central nervous system of rhesus monkeys. Signal Transduct Target Ther. 2021;6:169.
Burks SM, Rosas-Hernandez H, Alejandro Ramirez-Lee M, Cuevas E, Talpos JC. Can SARS-CoV-2 infect the central nervous system via the olfactory bulb or the blood-brain barrier? Brain Behav Immun. 2021;95:7–14.
Chu H, Chan JF, Yuen TT, Shuai H, Yuan S, Wang Y, et al. Comparative tropism, replication kinetics, and cell damage profiling of SARS-CoV-2 and SARS-CoV with implications for clinical manifestations, transmissibility, and laboratory studies of COVID-19: an observational study. Lancet Microbe. 2020;1:e14–e23.
Zhang BZ, Chu H, Han S, Shuai H, Deng J, Hu YF, et al. SARS-CoV-2 infects human neural progenitor cells and brain organoids. Cell Res. 2020;30:928–31.
Mesci P, de Souza JS, Martin-Sancho L, Macia A, Saleh A, Yin X, et al. SARS-CoV-2 infects human brain organoids causing cell death and loss of synapses that can be rescued by treatment with Sofosbuvir. PLoS Biol. 2022;20:e3001845.
Kettunen P, Lesnikova A, Rasanen N, Ojha R, Palmunen L, Laakso M, et al. SARS-CoV-2 infection of human neurons is TMPRSS2 independent, requires endosomal cell entry, and can be blocked by inhibitors of host phosphoinositol-5 kinase. J Virol. 2023;97:e0014423.
Krasemann S, Haferkamp U, Pfefferle S, Woo MS, Heinrich F, Schweizer M, et al. The blood-brain barrier is dysregulated in COVID-19 and serves as a CNS entry route for SARS-CoV-2. Stem Cell Reports. 2022;17:307–20.
Tang AT, Buchholz DW, Szigety KM, Imbiakha B, Gao S, Frankfurter M, et al. Cell-autonomous requirement for ACE2 across organs in lethal mouse SARS-CoV-2 infection. PLoS Biol. 2023;21:e3001989.
Nikiforuk AM, Kuchinski KS, Twa DDW, Lukac CD, Sbihi H, Basham CA, et al. The contrasting role of nasopharyngeal angiotensin converting enzyme 2 (ACE2) transcription in SARS-CoV-2 infection: a cross-sectional study of people tested for COVID-19 in British Columbia, Canada. EBioMedicine. 2021;66:103316.
Mpekoulis G, Frakolaki E, Taka S, Ioannidis A, Vassiliou AG, Kalliampakou KI, et al. Alteration of L-Dopa decarboxylase expression in SARS-CoV-2 infection and its association with the interferon-inducible ACE2 isoform. PLoS ONE. 2021;16:e0253458.
Fajnzylber J, Regan J, Coxen K, Corry H, Wong C, Rosenthal A, et al. SARS-CoV-2 viral load is associated with increased disease severity and mortality. Nat Commun. 2020;11:5493.
Boyapati A, Wipperman MF, Ehmann PJ, Hamon S, Lederer DJ, Waldron A, et al. Baseline severe acute respiratory syndrome viral load is associated with coronavirus disease 2019 severity and clinical outcomes: post hoc analyses of a phase 2/3 trial. J Infect Dis. 2021;224:1830–8.
Caceres PS, Savickas G, Murray SL, Umanath K, Uduman J, Yee J, et al. High SARS-CoV-2 viral load in urine sediment correlates with acute kidney injury and poor COVID-19 outcome. J Am Soc Nephrol. 2021;32:2517–28.
Paranjpe I, Chaudhary K, Johnson KW, Jaladanki SK, Zhao S, De Freitas JK, et al. Association of SARS-CoV-2 viral load at admission with in-hospital acute kidney injury: a retrospective cohort study. PLoS ONE. 2021;16:e0247366.
Hou YJ, Okuda K, Edwards CE, Martinez DR, Asakura T, Dinnon KH 3rd, et al. SARS-CoV-2 reverse genetics reveals a variable infection gradient in the respiratory tract. Cell. 2020;182:429–46.
Lim KH, Yang S, Kim SH, Joo JY. Elevation of ACE2 as a SARS-CoV-2 entry receptor gene expression in Alzheimer’s disease. J Infect. 2020;81:e33–e34.
Ding Q, Shults NV, Gychka SG, Harris BT, Suzuki YJ. Protein expression of angiotensin-converting enzyme 2 (ACE2) is upregulated in brains with Alzheimer’s disease. Int J Mol Sci. 2021;22:1687.
Zhao Y, Li W, Lukiw W. Ubiquity of the SARS-CoV-2 receptor ACE2 and upregulation in limbic regions of Alzheimer’s disease brain. Folia Neuropathol. 2021;59:232–8.
Reveret L, Leclerc M, Emond V, Tremblay C, Loiselle A, Bourassa P, et al. Higher angiotensin-converting enzyme 2 (ACE2) levels in the brain of individuals with Alzheimer’s disease. Acta Neuropathol Commun. 2023;11:159.
Mehri S, Finsterer J. A stroke in severe acute respiratory syndrome coronavirus 2 infected is not necessarily a COVID-stroke. Brain Circ. 2023;9:198–9.
Nagamine T. Restlessness with manic episodes induced by right-sided multiple strokes after COVID-19 infection: a case report. Brain Circ. 2023;9:112–5.
Nagamine T. Beware of bihemispheric stroke after Omicron variant infection in the elderly. Brain Circ. 2023;9:52–54.
Carpio-Orantes LD, Solis-Sanchez I, Moreno-Aldama NP, Aguilar-Silva A, Garcia-Mendez S, Sanchez-Diaz JS. Incidence of stroke in a population affected by COVID-19 in Veracruz, Mexico. Brain Circ. 2023;9:55–56.
Kurian C, Mayer S, Kaur G, Sahni R, Feldstein E, Samaan M, et al. Bihemispheric ischemic strokes in patients with COVID-19. Brain Circ. 2022;8:10–16.
Alhazmi FH, Alsharif WM, Alshoabi SA, Gameraddin M, Aloufi KM, Abdulaal OM, et al. Identifying cerebral microstructural changes in patients with COVID-19 using MRI: a systematic review. Brain Circ. 2023;9:6–15.
Wijnant SRA, Jacobs M, Van Eeckhoutte HP, Lapauw B, Joos GF, Bracke KR, et al. Expression of ACE2, the SARS-CoV-2 receptor, in lung tissue of patients with type 2 diabetes. Diabetes. 2020;69:2691–9.
Pinto BGG, Oliveira AER, Singh Y, Jimenez L, Goncalves ANA, Ogava RLT, et al. ACE2 expression is increased in the lungs of patients with comorbidities associated with severe COVID-19. J Infect Dis. 2020;222:556–63.
Jacobs M, Van Eeckhoutte HP, Wijnant SRA, Janssens W, Joos GF, Brusselle GG, et al. Increased expression of ACE2, the SARS-CoV-2 entry receptor, in alveolar and bronchial epithelium of smokers and COPD subjects. Eur Respir J. 2020;56:2002378.
Choi JY, Lee HK, Park JH, Cho SJ, Kwon M, Jo C, et al. Altered COVID-19 receptor ACE2 expression in a higher risk group for cerebrovascular disease and ischemic stroke. Biochem Biophys Res Commun. 2020;528:413–9.
Bristow MR, Zisman LS, Altman NL, Gilbert EM, Lowes BD, Minobe WA, et al. Dynamic regulation of SARS-Cov-2 binding and cell entry mechanisms in remodeled human ventricular myocardium. JACC Basic Transl Sci. 2020;5:871–83.
Chen L, Li X, Chen M, Feng Y, Xiong C. The ACE2 expression in human heart indicates new potential mechanism of heart injury among patients infected with SARS-CoV-2. Cardiovasc Res. 2020;116:1097–100.
Kehoe PG. Angiotensins and Alzheimer’s disease: a bench to bedside overview. Alzheimers Res Ther. 2009;1:3.
Abbasi J. Choose ARBs over ACE inhibitors for first-line hypertension treatment, large new analysis suggests. JAMA. 2021;326:1244–5.
Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID-19 infection? Lancet Respir Med. 2020;8:e21.
Ouk M, Wu CY, Rabin JS, Jackson A, Edwards JD, Ramirez J, et al. The use of angiotensin-converting enzyme inhibitors vs. angiotensin receptor blockers and cognitive decline in Alzheimer’s disease: the importance of blood-brain barrier penetration and APOE epsilon4 carrier status. Alzheimers Res Ther. 2021;13:43.
Ohrui T, Tomita N, Sato-Nakagawa T, Matsui T, Maruyama M, Niwa K, et al. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology. 2004;63:1324–5.
Gao Y, O’Caoimh R, Healy L, Kerins DM, Eustace J, Guyatt G, et al. Effects of centrally acting ACE inhibitors on the rate of cognitive decline in dementia. BMJ Open. 2013;3:e002881.
Sink KM, Leng X, Williamson J, Kritchevsky SB, Yaffe K, Kuller L, et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: results from the Cardiovascular Health Study. Arch Intern Med. 2009;169:1195–202.
Janson J, Laedtke T, Parisi JE, O’Brien P, Petersen RC, Butler PC. Increased risk of type 2 diabetes in Alzheimer disease. Diabetes. 2004;53:474–81.
McLachlan CS. The angiotensin-converting enzyme 2 (ACE2) receptor in the prevention and treatment of COVID-19 are distinctly different paradigms. Clin Hypertens. 2020;26:14.
Gheware A, Ray A, Rana D, Bajpai P, Nambirajan A, Arulselvi S, et al. ACE2 protein expression in lung tissues of severe COVID-19 infection. Sci Rep. 2022;12:4058.
Green R, Mayilsamy K, McGill AR, Martinez TE, Chandran B, Blair LJ, et al. SARS-CoV-2 infection increases the gene expression profile for Alzheimer’s disease risk. Mol Ther Methods Clin Dev. 2022;27:217–29.
Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14:133–50.
Paz Ocaranza M, Riquelme JA, Garcia L, Jalil JE, Chiong M, Santos RAS, et al. Counter-regulatory renin-angiotensin system in cardiovascular disease. Nat Rev Cardiol. 2020;17:116–29.
Evans CE, Miners JS, Piva G, Willis CL, Heard DM, Kidd EJ, et al. ACE2 activation protects against cognitive decline and reduces amyloid pathology in the Tg2576 mouse model of Alzheimer’s disease. Acta Neuropathol. 2020;139:485–502.
Imai Y, Kuba K, Rao S, Huan Y, Guo F, Guan B, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–6.
Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–9.
Mizrahi B, Shilo S, Rossman H, Kalkstein N, Marcus K, Barer Y, et al. Longitudinal symptom dynamics of COVID-19 infection. Nat Commun. 2020;11:6208.
Molteni E, Sudre CH, Canas LS, Bhopal SS, Hughes RC, Antonelli M, et al. Illness duration and symptom profile in symptomatic UK school-aged children tested for SARS-CoV-2. Lancet Child Adolesc Health. 2021;5:708–18.
Harb AA, Chen R, Chase HS, Natarajan K, Noble JM. Clinical features and outcomes of patients with dementia compared to an aging cohort hospitalized during the initial New York City COVID-19 wave. J Alzheimers Dis. 2021;81:679–90.
Li J, Long X, Huang H, Tang J, Zhu C, Hu S, et al. Resilience of Alzheimer’s disease to COVID-19. J Alzheimers Dis. 2020;77:67–73.
Kragstrup TW, Singh HS, Grundberg I, Nielsen AL, Rivellese F, Mehta A, et al. Plasma ACE2 predicts outcome of COVID-19 in hospitalized patients. PLoS ONE. 2021;16:e0252799.
Furuhashi M, Sakai A, Tanaka M, Higashiura Y, Mori K, Koyama M, et al. Distinct regulation of U-ACE2 and P-ACE2 (urinary and plasma angiotensin-converting enzyme 2) in a Japanese general population. Hypertension. 2021;78:1138–49.
Patel SK, Juno JA, Lee WS, Wragg KM, Hogarth PM, Kent SJ, et al. Plasma ACE2 activity is persistently elevated following SARS-CoV-2 infection: implications for COVID-19 pathogenesis and consequences. Eur Respir J. 2021;57:2003730.
Garcia-Ayllon MS, Moreno-Perez O, Garcia-Arriaza J, Ramos-Rincon JM, Cortes-Gomez MA, Brinkmalm G, et al. Plasma ACE2 species are differentially altered in COVID-19 patients. FASEB J. 2021;35:e21745.
Lundstrom A, Ziegler L, Havervall S, Rudberg AS, von Meijenfeldt F, Lisman T, et al. Soluble angiotensin-converting enzyme 2 is transiently elevated in COVID-19 and correlates with specific inflammatory and endothelial markers. J Med Virol. 2021;93:5908–16.
Fagyas M, Fejes Z, Suto R, Nagy Z, Szekely B, Pocsi M, et al. Circulating ACE2 activity predicts mortality and disease severity in hospitalized COVID-19 patients. Int J Infect Dis. 2022;115:8–16.
Ramchand J, Burrell LM. Circulating ACE2: a novel biomarker of cardiovascular risk. Lancet. 2020;396:937–9.
Narula S, Yusuf S, Chong M, Ramasundarahettige C, Rangarajan S, Bangdiwala SI, et al. Plasma ACE2 and risk of death or cardiometabolic diseases: a case-cohort analysis. Lancet. 2020;396:968–76.
Kehoe PG, Wong S, Al Mulhim N, Palmer LE, Miners JS. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-beta and tau pathology. Alzheimers Res Ther. 2016;8:50.
Rockx B, Kuiken T, Herfst S, Bestebroer T, Lamers MM, Oude Munnink BB, et al. Comparative pathogenesis of COVID-19, MERS, and SARS in a nonhuman primate model. Science. 2020;368:1012–5.
Andrews MG, Mukhtar T, Eze UC, Simoneau CR, Ross J, Parikshak N, et al. Tropism of SARS-CoV-2 for human cortical astrocytes. Proc Natl Acad Sci USA. 2022;119:e2122236119.
Baggen J, Jacquemyn M, Persoons L, Vanstreels E, Pye VE, Wrobel AG, et al. TMEM106B is a receptor mediating ACE2-independent SARS-CoV-2 cell entry. Cell. 2023;186:3427–42.
Yang AC, Vest RT, Kern F, Lee DP, Agam M, Maat CA, et al. A human brain vascular atlas reveals diverse mediators of Alzheimer’s risk. Nature. 2022;603:885–92.
Magusali N, Graham AC, Piers TM, Panichnantakul P, Yaman U, Shoai M, et al. A genetic link between risk for Alzheimer’s disease and severe COVID-19 outcomes via the OAS1 gene. Brain. 2021;144:3727–41.
Zhang Y, Xu F, Wang T, Han Z, Shang H, Han K, et al. Shared genetics and causal association between plasma levels of SARS-CoV-2 entry receptor ACE2 and Alzheimer’s disease. CNS Neurosci Ther. 2024;30:e14873.
Acknowledgements
We thank the Genotype-Tissue Expression (GTEx) Project. The Genotype-Tissue Expression (GTEx) Project was supported by the Common Fund of the Office of the Director of the National Institutes of Health, and by NCI, NHGRI, NHLBI, NIDA, NIMH, and NINDS. The data used for the analyses described in this manuscript were obtained from: https://www.gtexportal.org/home/ the GTEx Portal (GTEx Analysis Release V10 (dbGaP Accession phs000424.v10.p2)) on December 4, 2024.
Funding
This work was supported by funding from the National Key R&D Program of China (No. 2023YFC3605200, and 2023YFC3605202), National Science and Technology Major Project (2023ZD0505306), National Natural Science Foundation of China (No. 82471449, 82071212, and 82371305), Beijing Natural Science Foundation (No. JQ21022 and Z240021).
Author information
Authors and Affiliations
Contributions
GYL, SJL, JYS, PGY and MXW conceived and initiated the project. GYL, HL, SJL, JYS, ZFH, TW, SG, PZ, and YC systematically searched PubMed, Google Scholar, Web of Science, Scopus, and Embase to identify the potential articles, analyzed the data, and wrote the first draft of the manuscript. All authors contributed to the interpretation of the results and critical revision of the manuscript for important intellectual content and approved the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This article contains human participants collected by several studies performed by previous studies. All participants gave informed consent in all the corresponding original studies. Here, our study is based on the publicly available datasets, and not the individual-level data. Hence, ethical approval was not sought.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, S., Sun, J., Li, H. et al. Expression of SARS-CoV-2 entry receptor ACE2 in human brain and its association with Alzheimer’s disease and COVID-19. Mol Psychiatry 30, 3257–3268 (2025). https://doi.org/10.1038/s41380-025-03006-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41380-025-03006-z