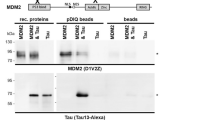
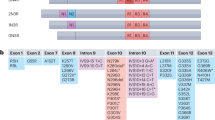
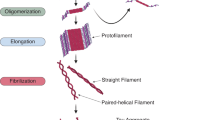
Abstract
Injury responses in terminally differentiated cells such as neurons are tightly regulated by pathways aiding homeostatic maintenance. Cancer patients subjected to neuronal injury in brain radiation experience cognitive declines similar to those seen in primary neurodegenerative diseases. Numerous studies have investigated the effect of radiation in proliferating cells of the brain, yet the impact in differentiated, post-mitotic neurons, especially the structural and functional alterations remain largely elusive. We identified that microtubule-associated tau is a critical player in neuronal injury response via compartmentalized functions in both repair-centric and synaptic regulatory pathways. Ionizing radiation-induced injury acutely induces an increase in phosphorylated tau in the nucleus where it directly interacts with histone 2AX (H2AX), a DNA damage repair (DDR) marker. Loss of tau significantly reduced H2AX phosphorylation after irradiation, indicating that tau may play an important role in the neuronal DDR response. We also observed that loss of tau increases eukaryotic elongation factor levels, a positive regulator of protein translation after irradiation. This initial response cascades into a significant increase in synaptic proteins, resulting in disrupted homeostasis. Downstream, the novel object recognition test showed a decrease in learning and memory in tau-knockout mice after irradiation, and electroencephalographic activity contained increased delta and theta band oscillations, often seen in dementia patients. Our findings demonstrate tau’s previously undefined, multifunctional role in acute responses to injury, ranging from DDR response in the nucleus to synaptic function within neurons. Such knowledge is vital to develop therapeutic strategies targeting neuronal injury in cognitive decline for at risk and vulnerable populations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
All relevant data are included in the paper. Data are available from the corresponding author upon reasonable request.
References
Davis FG, Dolecek TA, McCarthy BJ, Villano JL. Toward determining the lifetime occurrence of metastatic brain tumors estimated from 2007 United States cancer incidence data. Neuro Oncol. 2012;14:1171–7.
Hossain MJ, Xiao W, Tayeb M, Khan S. Epidemiology and prognostic factors of pediatric brain tumor survival in the US: evidence from four decades of population data. Cancer Epidemiol. 2021;72:101942. https://doi.org/10.1016/j.canep.2021.101942
Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, II’yasova D, et al. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–68.
Gould J. Breaking down the epidemiology of brain cancer. Nature. 2018;561:S40–S41.
Ostrom QT, Gittleman H, Farah P, Ondracek A, Chen Y, Wolinsky Y, et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2006-2010. Neuro Oncol. 2013;15:ii1–ii56.
DeNunzio NJ, Yock TI. Modern radiotherapy for pediatric brain tumors. Cancers. 2020;12:1533.
Grunert M, Kassubek R, Danz B, Klemenz B, Hasslacher S, Stroh S, et al. Radiation and brain tumors: an overview. Crit Rev Oncog. 2018;23:119–38.
Baumann M, Krause M, Overgaard J, Debus J, Bentzen AM, Daartz J, et al. Radiation oncology in the era of precision medicine. Nat Rev Cancer. 2016;16:234–49.
Mehta MP, Tomé WA, Olivera GH. Radiotherapy for brain tumors. Curr Oncol Rep. 2000;2:438–44.
Hoffman KE, Yock TI. Radiation therapy for pediatric central nervous system tumors. J Child Neurol. 2009;24:1387–96.
Salari N, Ghasemi H, Fatahian R, Mansouri K, Dokaneheifard S, Shiri MH, et al. The global prevalence of primary central nervous system tumors: a systematic review and meta-analysis. Eur J Med Res. 2023;28:39.
Ramírez-Guerrero S, Vargas-Cuellar MP, Charry-Sánchez JD, Talero-Gutiérrez C. Cognitive sequelae of radiotherapy in primary brain tumors. Interdiscip Neurosurg. 2022;98:18.
Al Dahhan NZ, Cox E, Nieman BJ, Mabbott DJ. Cross-translational models of late-onset cognitive sequelae and their treatment in pediatric brain tumor survivors. Neuron. 2022;110:2215–41.
Michaelidesová A, Konířová J, Bartůněk P, Zíková M. Effects of radiation therapy on neural stem cells. Genes. 2019;10:640.
Mineyeva OA, Bezriadnov DV, Kedrov AV, Lazutkin AA, Anokhin KV, Enikolopov GN. Radiation induces distinct changes in defined subpopulations of neural stem and progenitor cells in the adult hippocampus. Front Neurosci. 2018;12:1013.
Wu PH, Coultrap S, Pinnix C, Davies KD, Tailor R, Ang KK, et al. Radiation induces acute alterations in neuronal function. PLoS ONE. 2012;7:e37677. https://doi.org/10.1371/journal.pone.0037677
Zhang D, Zhou W, Lam TT, Li Y, Duman JG, Dougherty PM, et al. Cranial irradiation induces axon initial segment dysfunction and neuronal injury in the prefrontal cortex and impairs hippocampal coupling. Neurooncol Adv. 2020;2:vdaa058.
Greene-Schloesser D, Robbins ME, Peiffer AM, Shaw EG, Wheeler KT, Chan MD. Radiation-induced brain injury: a review. Front Oncol. 2012;2:73.
Balentova S, Adamkov M. Molecular, cellular and functional effects of radiation-induced brain injury: a review. Int J Mol Sci. 2015;16:27796–815.
Xiong M, Tao Y, Gao Q, Feng B, Yan W, Zhou Y, et al. Human stem cell-derived neurons repair circuits and restore neural function. Cell Stem Cell. 2021;28:112–26.
Luo L. Architectures of neuronal circuits. Science. 2021;373:eabg7285.
Ünal HT, Başçiftçi F. Evolutionary design of neural network architectures: a review of three decades of research. Artif Intell Rev. 2022;55:1723–802.
Schmidt ERE, Polleux F. Genetic mechanisms underlying the evolution of connectivity in the human cortex. Front Neural Circuits. 2022;15:787164.
Saxton WM, Hollenbeck PJ. The axonal transport of mitochondria. J Cell Sci. 2006;125:2095–104.
Vona R, Mileo AM, Matarrese P. Microtubule-based mitochondrial dynamics as a valuable therapeutic target in cancer. Cancers. 2021;13:5812.
Lasser M, Tiber J, Lowery LA. The role of the microtubule cytoskeleton in neurodevelopmental disorders. Front Cell Neurosci. 2018;12:165.
Parato J, Bartolini F. The microtubule cytoskeleton at the synapse. Neurosci Lett. 2022;753:135850.
Takei Y, Kikkawa YS, Atapour N, Hensch TK, Hirokawa N. Defects in synaptic plasticity, reduced NMDA-receptor transport, and instability of postsynaptic density proteins in mice lacking microtubule-associated protein 1A. J Neurosci. 2015;35:15539–54.
Bodakuntla S, Jijumon AS, Villablanca C, Gonzalez-Billault C, Janke C. Microtubule-associated proteins: structuring the cytoskeleton. Trends Cell Biol. 2019;2:804–19.
Méphon-Gaspard A, Boca M, Pioche-Durieu C, Desforges B, Burgo A, Hamon L, et al. Role of tau in the spatial organization of axonal microtubules: keeping parallel microtubules evenly distributed despite macromolecular crowding. Cell Mol Life Sci. 2016;73:3745–60.
Cario A, Berger CL. Tau, microtubule dynamics, and axonal transport: new paradigms for neurodegenerative disease. Bioessays. 2023;45:e2200138.
Ulrich G, Salvade A, Boersema P, Cali T, Foglieni C, Sola M, et al. Phosphorylation of nuclear tau is modulated by distinct cellular pathways. Sci Rep. 2018;8:17702.
Violet M, Delattre L, Tardivel M, Sultan A, Chauderlier A, Cailierez R, et al. A major role for tau in neuronal DNA and RNA protection in vivo under physiological and hyperthermic conditions. Front Cell Neurosci. 2014;8:84.
Sultan A, Nesslany F, Violet M, Begard S, Loyens A, Talahari S, et al. Nuclear tau, a key player in neuronal DNA protection. J Biol Chem. 2011;286:4566–75.
Asada-Utsugi M, Uemura K, Ayaki T, Uemura MT, Minamiyama S, Hikiami R, et al. Failure of DNA double-strand break repair by tau mediates Alzheimer’s disease pathology in vitro. Commun Biol. 2022;5:358.
Mietelska-Porowska A, Wasik U, Goras M, Filipek A, Niewiadomska G. Tau protein modifications and interactions: their role in function and dysfunction. Int J Mol Sci. 2014;15:4671–713.
Barbier P, Zejneli O, Martinho M, Lasorsa A, Belle V, Smet-Nocca C, et al. Role of tau as a microtubule-associated protein: structural and functional aspects. Front Aging Neurosci. 2019;11:204.
He HJ, Wang XS, Pan R, Wang DL, Liu MN, He RQ. The proline-rich ___domain of tau plays a role in interactions with actin. BMC Cell Biol. 2009;10:81.
Benhelli-Mokrani H, Mansoroglu Z, Chauderlier A, Albaud B, Gentien D, Sommer S, et al. Genome-wide identification of genic and intergenic neuronal DNA regions bound by Tau protein under physiological and stress conditions. Nucleic Acids Res. 2018;46:11405–22.
Azzam EI, Jay-Gerin JP, Pain D. Ionizing radiation-induced metabolic oxidative stress and prolonged cell injury. Cancer Lett. 2012;327:48–60.
Huang R-X, Zhou P-K. DNA damage response signaling pathways and targets for radiotherapy sensitization in cancer. Signal Transduct Target Ther. 2020;5:60.
Wang W-Y, Pan L, Su SC, Quinn EJ, Sasaki M, Jimenez JC, et al. Interaction of FUS and HDAC1 regulates DNA damage response and repair in neurons. Nat Neurosci. 2013;16:1383–91.
Tolias KF, Bikoff JB, Burette A, Paradis S, Harrar D, Tavazoie S, et al. The Rac1-GEF Tiam1 couples the NMDA receptor to the activity-dependent development of dendritic arbors and spines. Neuron. 2005;45:525–38.
Duman JG, Tzeng CP, Tu Y-K, Munjal T, Schwechter B, Ho TS-Y, et al. The adhesion-GPCR BAI1 regulates synaptogenesis by controlling the recruitment of the Par3/Tiam1 polarity complex to synaptic sites. J Neurosci. 2013;33:6964–78.
Bankhead, P. et al. QuPath: Open source software for digital pathology image analysis. Sci Rep. 2017;7:16878.
Villasana LE, Klann E, Tejada-Simon MV. Rapid isolation of synaptoneurosomes and postsynaptic densities from adult mouse hippocampus. J Neurosci Methods. 2006;158:30–6.
Goodman CA, Mabrey DM, Frey JW, Miu MH, Schmidt EK, Pierre P, et al. Novel insights into the regulation of skeletal muscle protein synthesis as revealed by a new nonradioactive in vivo technique. FASEB J. 2011;3:25.
Leger M, Quiedeville A, Bouet V, Haelewyn B, Boulouard M, Schumann-Bard P, et al. Object recognition test in mice. Nat Protoc. 2013;8:2531–7.
Noble W, Hange DP, Miller CCJ, Lovestone S. The importance of tau phosphorylation for neurodegenerative diseases. Front Neurol. 2013;4:83.
Roqanian S, Ahmadian S, Nabavi SM, Pakdaman H, Shafiezadeh M, Goudarzi G, et al. Tau nuclear translocation is a leading step in tau pathology process through P53 stabilization and nucleolar dispersion. J Neurosci Res. 2022;100:1084–104.
Averbeck D, Rodriguez-Lafrasse C. Role of mitochondria in radiation responses: epigenetic, metabolic, and signaling impacts. Int J Mol Sci. 2021;22:11047.
Davydova E, Ho AYY, Malecki J, Moen A, Enserink JM, Jakobsson ME, et al. Identification and characterization of a novel evolutionarily conserved lysine-specific methyltransferase targeting eukaryotic translation elongation factor 2 (eEF2). J Biol Chem. 2014;289:30499–510.
Beretta S, Gritti L, Verpelli C, Sala C. Eukaryotic elongation factor 2 kinase a pharmacological target to regulate protein translation dysfunction in neurological diseases. Neuroscience. 2020;445:42–49.
Ma T. Roles of eukaryotic elongation factor 2 kinase (eEF2K) in neuronal plasticity, cognition, and Alzheimer’s disease. Acta Neuropathol. 2023;166:47–57.
Goodman CA, Hornberger TA. Measuring protein synthesis with SUnSET: a valid alternative to traditional techniques? Exerc Sport Sci Rev. 2013;41:107–15.
Sutton MA, Taylor AM, Ito HT, Pham A, Schuman EM. Postsynaptic decoding of neural activity: eEF2 as a biochemical sensor coupling miniature synaptic transmission to local protein synthesis. Neuron. 2007;55:648–61.
Marx M-C, Billups D, Billups B. Maintaining the presynaptic glutamate supply for excitatory neurotransmission. J Neurosci Res. 2015;93:1031–44.
Pal MM. Glutamate: the master neurotransmitter and its implications in chronic stress and mood disorders. Front Hum Neurosci. 2021;15:722323.
Stevens CF, Williams JH. “Kiss and run” exocytosis at hippocampal synapses. Proc Natl Acad Sci USA. 2000;97:12828–33.
Hoopmann P, Rizzoli SO, Betz WJ. Imaging synaptic vesicle recycling by staining and destaining vesicles with FM dyes. Cold Spring Harb Protoc. 2012;1:77–83.
Kavalali ET, Klingauf J, Tsien RW. Properties of fast endocytosis at hippocampal synapses. Philos Trans R Soc Lond B Biol Sci. 1999;354:337–46.
Lueptow LM. Novel object recognition test for the investigation of learning and memory in mice. J Vis Exp. 2017;126:55718.
Yang S-T, Shi Y, Wang Q, Peng J-Y, Li B-M. Neuronal representation of working memory in the medial prefrontal cortex of rats. Mol Brain. 2017;7:61.
Zawiślak-Fornagiel K, Ledwon D, Bugdol M, Romaniszyn-Kania P, Malecki A, Gorzkowska A, et al. The increase of theta power and decrease of alpha/theta ratio as a manifestation of cognitive impairment in Parkinson’s disease. J Clin Med. 2023;12:12041569.
Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer’s disease, dementia with lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain. 2008;131:690–705.
Sokolov MV, Smilenov LB, Hall EJ, Panyutin IG, Bonner WM, Sedelnikova OA. Ionizing radiation induces DNA double-strand breaks in bystander primary human fibroblasts. Oncogene. 2005;24:7257–65.
Iliakis G, Wang Y, Guan J, Wang H. DNA damage checkpoint control in cells exposed to ionizing radiation. Oncogene. 2003;22:5834–47.
Mansuroglu Z, Benhelli-Mokrani H, Marcato V, Sultan A, Violet M, Chauderlier A, et al. Loss of tau protein affects the structure, transcription and repair of neuronal pericentromeric heterochromatin. Sci Rep. 2016;6:33047.
Schwab N, Tator C, Hazrati LN. DNA damage as a marker of brain damage in individuals with history of concussions. Lab Invest. 2019;99:1008–18.
Portillo M, Eremenko E, Kaluski S, Garcia-Venzor A, Onn L, Stein D, et al. SIRT6-CBP-dependent nuclear tau accumulation and its role in protein synthesis. Cell Rep. 2021;35:109035.
Wu CI, Wen H. Heightened protein-translation activities in mammalian cells and the disease/treatment implications. Natl Sci Rev. 2020;7:1851–5.
Roux PP, Topisirovic I. Regulation of mRNA translation by signaling pathways. Cold Spring Harb Perspect Biol. 2012;4:a012252.
Kapur M, Monaghan CE, Ackerman SL. Regulation of mRNA translation in Neurons-A matter of life and death. Neuron. 2017;96:616–37.
Kindler S, Kreienkamp HJ. Dendritic mRNA targeting and translation. Adv Exp Med Biol. 2012;970:285–305.
Bramham CR, Wells DG. Dendritic mRNA: transport, translation and function. Nat Rev Neurosci. 2007;8:776–89.
Schuman EM, Dynes JL, Steward O. Synaptic regulation of translation of dendritic mRNAs. J Neurosci. 2006;26:7143–6.
Mofatteh M. mRNA localization and local translation in neurons. AIMS Neurosci. 2020;7:299–310.
Sossin WS, Costa-Mattioli M. Translational control in the brain in health and disease. Cold Spring Harb Perspect Biol. 2019;11:a032912.
Wang S, Sun S. Translation dysregulation in neurodegenerative diseases: a focus on ALS. Mol Neurodegener. 2023;18:58.
Brilkova M, Nigri M, Kumar HS, Moore J, Mantovani M, Keller C, et al. Error-prone protein synthesis recapitulates early symptoms of Alzheimer disease in aging mice. Cell Rep. 2022;40:111433.
Buffington SA, Huang W, Costa-Mattioli M. Translational control in synaptic plasticity and cognitive dysfunction. Annu Rev Neurosci. 2014;37:17–38.
Heise C, Taha E, Murru L, Ponzoni L, Cattaneo A, Guarnieri FC, et al. eEF2K/eEF2 pathway controls the excitation/inhibition balance and susceptibility to epileptic seizures. Cereb Cortex. 2017;27:2226–48.
Taha E, Gildish I, Gal-Ben-Ari S, Rosenblum K. The role of eEF2 pathway in learning and synaptic plasticity. Neurobiol Learn Mem. 2013;105:100–6.
Verpelli C, Piccoli G, Zibetti C, Zanchi A, Gardoni F, Huang K, et al. Synaptic activity controls dendritic spine morphology by modulating eEF2-dependent BDNF synthesis. J Neurosci. 2010;30:5830–42.
Parihar VK, Limoli CL. Cranial irradiation compromises neuronal architecture in the hippocampus. Proc Natl Acad Sci USA. 2013;110:12822–7.
Harada A, Oguchi K, Okabe S, Kuno J, Terada S, Ohshima T, et al. Altered microtubule organization in small-calibre axons of mice lacking tau protein. Nature. 1994;369:488–91.
Duman JG, Dinh J, Zhou W, Cham H, Mavratsas VC, Paveskovic M, et al. Memantine prevents acute radiation-induced toxicities at hippocampal excitatory synapses. Neuro Oncol. 2020;20:655–65.
Liu CW, Lee G, Jay DG. Tau is required for neurite outgrowth and growth cone motility of chick sensory neurons. Cell Motil Cytoskeleton. 1999;43:232–42.
Biswas S, Kalil K. The microtubule-associated protein tau mediates the organization of microtubules and their dynamic exploration of actin-rich lamellipodia and filopodia of cortical growth cones. J Neurosci. 2018;38:291–307.
Dawson HN, Ferreira A, Eyster MV, Ghoshal N, Binder LI, Vitek MP. Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. J Cell Sci. 2001;114:1179–87.
Acknowledgements
We are grateful to University of Texas Medical Branch, especially Dr. Aaron Bailey for the IP-MS assay and preliminary analysis, and ActiveMotif for the ChIP-Seq assay and analysis. This study was supported by funding from the National Institute of Health (R01CA256848, R01CA208535, R01CA255596, and R01CA219667). Figure illustrations were generated on BioRender.
Author information
Authors and Affiliations
Contributions
Conceptualization, Riya T and DRG; Methodology, Riya T; Investigation, Riya T, DZ, CAC, Rintu T, SKS, LFB; Writing – Original Draft, Riya T; Writing – Review & Editing, Riya T, DZ, CAC, Rintu T, SKS, LFB, RFM, JGD, DRG; Funding Acquisition, DRG; Resources, DRG; Supervision, DRG.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods were conducted in accordance with institutional guidelines and regulations. No human participants were involved in this study. Studies involving mice were approved by The University of Texas Institutional Animal Care and Use Committee.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Thomas, R., Zhang, D., Cronkite, C.A. et al. Subcellular functions of tau mediate repair response and synaptic homeostasis in injury. Mol Psychiatry (2025). https://doi.org/10.1038/s41380-025-03029-6
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41380-025-03029-6