Abstract
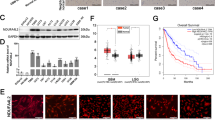
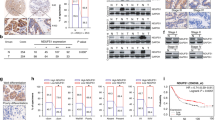
Hypoxia is a common feature of lung squamous cell carcinoma (LUSC), and hypoxia-inducible factor-1 (HIF-1) overexpression is associated with poor clinical outcome in LUSC. NADH dehydrogenase 1 alpha subcomplex subunit 4-like 2 (NDUFA4L2) is a recently identified target of HIF-1, but its roles in LUSC remain unclear. Herein, the expression and regulatory mechanisms of NDUFA4L2 were investigated in LUSC, and the influences on LUSC cell oxidative metabolism and survival of NDUFA4L2 were determined. The potential microRNA targeting to NDUFA4L2 was identified and its roles on LUSC cell were detected. We found that NDUFA4L2 were overexpressed in LUSC tissues, and that NDUFA4L2 expression correlated with shorter overall survival. NDUFA4L2 was regulated by HIF-1α under hypoxia, and NDUFA4L2 decreased mitochondrial reactive oxygen species (mitoROS) production through inhibiting mitochondrial complex I activity in LUSC cells. NDUFA4L2 silencing effectively suppressed LUSC cell growth and enhanced apoptosis by inducing mitoROS accumulation. Additionally, NDUFA4L2 was a target for miR-183-5p, and LUSC patients with high miR-183-5p levels had better prognoses. MiR-183-5p significantly induced mitoROS production and suppressed LUSC survival through negatively regulating NDUFA4L2 in vitro and in vivo. Our results suggested that regulation of NDUFA4L2 by HIF-1α is an important mechanism promoting LUSC progression under hypoxia. NDUFA4L2 inhibition using enforced miR-183-5p expression might be an effective strategy for LUSC treatment.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
References
Ruiz-Ceja KA, Chirino YI. Current FDA-approved treatments for non-small cell lung cancer and potential biomarkers for its detection. Biomed Pharmacother. 2017;90:24–37.
Zhao W, Choi YL, Song JY, Zhu Y, Xu Q, Zhang F, et al. ALK, ROS1 and RET rearrangements in lung squamous cell carcinoma are very rare. Lung Cancer. 2016;94:22–7.
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M, et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol. 2010;28:1835–42.
Johnson DH, Fehrenbacher L, Novotny WF, Herbst RS, Nemunaitis JJ, Jablons DM, et al. Randomized phase II trial comparing bevacizumab plus carboplatin and paclitaxel with carboplatin and paclitaxel alone in previously untreated locally advanced or metastatic non-small-cell lung cancer. J Clin Oncol. 2004;22:2184–91.
Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–33.
Herbst RS, Baas P, Kim D-W, Felip E, Pérez-Gracia JL, Han J-Y, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–50.
Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA Jr, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13:165–83.
Al Tameemi W, Dale TP, Al-Jumaily RMK, Forsyth NR. Hypoxia-modified cancer cell metabolism. Front Cell Devel Biol. 2019;7:4.
Bhandari V, Hoey C, Liu LY, Lalonde E, Ray J, Livingstone J, et al. Molecular landmarks of tumor hypoxia across cancer types. Nat Genet. 2019;51:308–18.
Xiang L, Semenza GL. Hypoxia-inducible factors promote breast cancer stem cell specification and maintenance in response to hypoxia or cytotoxic chemotherapy. Adv Cancer Res. 2019;141:175–212.
Nagao A, Kobayashi M, Koyasu S, Chow CCT, Harada H. HIF-1-dependent reprogramming of glucose metabolic pathway of cancer cells and its therapeutic significance. Int J Mol Sci. 2019;20:238.
Berezowska S, Galvan JA, Langer R, Bubendorf L, Savic S, Gugger M, et al. Glycine decarboxylase and HIF-1alpha expression are negative prognostic factors in primary resected early-stage non-small cell lung cancer. Virchows Arch. 2017;470:323–30.
Takasaki C, Kobayashi M, Ishibashi H, Akashi T, Okubo K. Expression of hypoxia-inducible factor-1alpha affects tumor proliferation and antiapoptosis in surgically resected lung cancer. Mol Clin Oncol. 2016;5:295–300.
Ren W, Mi D, Yang K, Cao N, Tian J, Li Z, et al. The expression of hypoxia-inducible factor-1alpha and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss Med Wkly. 2013;143:w13855.
Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–34.
Fukuda R, Zhang H, Kim JW, Shimoda L, Dang CV, Semenza GL. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell. 2007;129:111–22.
Semenza Gregg L. Oxygen-dependent regulation of mitochondrial respiration by hypoxia-inducible factor 1. Biochem J. 2007;405:1–9.
Tello D, Balsa E, Acosta-Iborra B, Fuertes-Yebra E, Elorza A, Ordonez A, et al. Induction of the mitochondrial NDUFA4L2 protein by HIF-1alpha decreases oxygen consumption by inhibiting Complex I activity. Cell Metab. 2011;14:768–79.
Lai RK, Xu IM, Chiu DK, Tse AP, Wei LL, Law CT, et al. NDUFA4L2 fine-tunes oxidative stress in hepatocellular Carcinoma. Clin Cancer Res. 2016;22:3105–17.
Laursen KB, Chen Q, Khani F, Attarwala N, Gross SS, Dow L, et al. Mitochondrial Ndufa4l2 Enhances Deposition of Lipids and expression of Ca9 in the TRACK model of early clear cell renal cell carcinoma. Front Oncol. 2021;11:783856.
Minton DR, Fu L, Mongan NP, Shevchuk MM, Nanus DM, Gudas LJ. Role of NADH dehydrogenase (Ubiquinone) 1 Alpha subcomplex 4-Like 2 in clear cell renal cell carcinoma. Clin Cancer Res. 2016;22:2791–801.
Zhou L, Mao LH, Li X, Wang QL, Chen SY, Chen ZJ, et al. Transcriptional regulation of NDUFA4L2 by NFIB induces sorafenib resistance by decreasing reactive oxygen species in hepatocellular carcinoma. Cancer Sci. 2023;114:793.
Kubala JM, Laursen KB, Schreiner R, Williams RM, van der Mijn JC, Crowley MJ, et al. NDUFA4L2 reduces mitochondrial respiration resulting in defective lysosomal trafficking in clear cell renal cell carcinoma. Cancer Biol Therapy. 2023;24:2170669.
Tang Z, Kang B, Li C, Chen T, Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019;47:W556–W60.
Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CML, et al. Targeting hypoxia to improve non-small cell lung cancer outcome. J Natl Cancer Inst. 2018;110 https://doi.org/10.1093/jnci/djx160.
Esterházy D, King MS, Yakovlev G, Hirst J. Production of reactive oxygen species by complex I (NADH: ubiquinone oxidoreductase) from Escherichia coli and comparison to the enzyme from mitochondria. Biochemistry. 2008;47:3964–71.
Nakamura H, Takada K. Reactive oxygen species in cancer: current findings and future directions. Cancer Sci. 2021;112:3945–52.
Checa J, Aran JM. Reactive oxygen species: drivers of physiological and pathological processes. J Inflammat Res. 2020;13:1057–73.
Sgarbi G, Gorini G, Liuzzi F, Solaini G, Baracca A. Hypoxia and IF1 expression promote ROS decrease in cancer cells. Cells. 2018;7:64.
Hayes JD, Dinkova-Kostova AT, Tew KD. Oxidative stress in cancer. Cancer Cell. 2020;38:167–97.
Garofalo M, Croce CM. microRNAs: master regulators as potential therapeutics in cancer. Annu Rev Pharmacol Toxicol. 2011;51:25–43.
Kudo M, Zalles N, Distefano R, Nigita G, Veneziano D, Gasparini P, et al. Synergistic apoptotic effect of miR-183-5p and Polo-Like kinase 1 inhibitor NMS-P937 in breast cancer cells. Cell Death Differ 2021;29:407–19.
Shen G, Li X, Jia Y-f, Piazza GA, Xi Y. Hypoxia-regulated microRNAs in human cancer. Acta Pharmacol Sin. 2013;34:336–41.
Tapeh BE-G, Alivand MR, Solalii S. Potential Interactions between miRNAs and hypoxia: a new layer in cancer hypoxia. Anti-Cancer Agents Med Chem. 2021;21:2315–26.
Liang Y, Wang T, Gao R, Jia X, Ji T, Shi P, et al. Fucosyltransferase 8 is overexpressed and influences clinical outcomes in lung adenocarcinoma patients. Pathol Oncol Res. 2022;28:1610116.
Zhu X, Zhang Y, Wang Y, Zhang H, Wang X, Tang H, et al. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine. 2022;96:153807.
He W, Zhang XY, Gong X, Luo K, Mao X, Lu E, et al. Drug‐free biomimetic oxygen supply nanovehicle promotes ischemia‐reperfusion therapy in stroke. Adv Funct Mater. 2023;33:2212919.
Chen S-j, Hoffman NE, Shanmughapriya S, Bao L, Keefer K, Conrad K, et al. A splice variant of the human ion channel TRPM2 modulates neuroblastoma tumor growth through hypoxia-inducible factor (HIF)-1/2α. J Biol Chem. 2014;289:36284–302.
Tahrir FG, Shanmughapriya S, Ahooyi TM, Knezevic T, Gupta MK, Kontos CD, et al. Dysregulation of mitochondrial bioenergetics and quality control by HIV‐1 Tat in cardiomyocytes. J Cell Physiol. 2018;233:748–58.
Li R, Shen Q, Wu N, He M, Liu N, Huang J, et al. MiR-145 improves macrophage-mediated inflammation through targeting Arf6. Endocrine. 2018;60:73–82.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (grant number 81902322), Natural Science Basic Research Program of Shaanxi Province (grant number 2020JQ-526 and 2023-JC-YB-791), Fundamental Research Funds for the Central Universities in Xi’an Jiaotong University (grant number xzy012021064).
Author information
Authors and Affiliations
Contributions
All authors have read the journal’s authorship agreement and that the manuscript has been reviewed by and approved by all named authors. Yiqian Liang, Aimin Yang and Boxiang Zhang conceived the study, designed the experiments, and acquired the financial support; Peng Han, Yixing Li and Aomei Zhao carried out cell culture and in vitro studies; Xinru Li, Hui Ren and Puyu Shi performed the in vivo studies and acquired data; Rui Gao and Jianjun Xue performed the database analyses; Peng Han, Yiqian Liang and Boxiang Zhan analyzed the data and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All methods in this study were performed in accordance with the Declaration of Helsinki and its subsequent revisions. Tissue microarray of human LUSC and paired adjacent normal tissues (HLugS180Su02) were purchased from Shanghai Outdo Biotech Company. The study was approved by the Ethics Committee of Shanghai Outdo Biotech Company (SHYJS-CP-1910013). All samples were obtained with patient’s informed content. The tissue samples for RT-PCR were collected at the First Affiliated Hospital of Xi’an Jiaotong University. The study was approved by the Ethic Committee of the First Affiliated Hospital of Xi’an Jiaotong University (No. XJTU1AF2020LSK-199) and informed consent was obtained from every participant. All animal studies were approved by the Institutional Animal Care and Use Committee of Xi’an Jiaotong University (No. 2018-025).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Han, P., Zhang, B., Li, Y. et al. MiR-183-5p inhibits lung squamous cell carcinoma survival through disrupting hypoxia adaptation mediated by HIF-1α/NDUFA4L2 axis. Oncogene 43, 2821–2834 (2024). https://doi.org/10.1038/s41388-024-03129-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-03129-7
This article is cited by
-
MiR-192-5p protects hexavalent chromium-induced apoptosis in renal epithelial cells by inhibiting p53 signaling pathway
Molecular & Cellular Toxicology (2025)