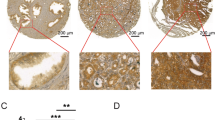
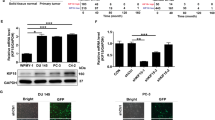
Abstract
Prostate is a zinc rich organ and the physiological function of the abundant zinc ions is relatively less understood. AKT kinase is a pivotal regulator downstream of cytokines, growth factors and other extracellular stimuli, and the attachment of its PH ___domain to PtdIns-3,4,5-P3 (PIP3) and the subsequent phosphorylation of its kinase ___domain by PDPK1 are considered important for its activation. Herein, we report a regulatory mechanism of AKT kinase by zinc ions. Mechanistically, zinc ions directly bind to AKT and facilitate AKT activation through disrupting the interaction between PH and kinase domains within AKT molecule. Consistently, AKT1-H89A/E91A mutant (zinc-binding-deficient) fails to respond to zinc ions and exhibits strong interaction between PH and kinase domains, and it is less oncogenic in orthotopic xenograft model of prostate cancer. On the other hand, the AKT1-W80L mutant with minimum intra-molecular interaction between PH and kinase domains shows strong tumor promoting capacity although it could not be further stimulated by zinc ions. Overall, this study reveals a distinctive regulatory mechanism of AKT activation and implies a tumor promoting role of the zinc ions in prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
This study does not report any original code. All data generated in this study are presented in this paper and the Supplementary Materials files. Any information supporting this article is available from the lead contact upon reasonable request.
References
McNeal JE. Normal histology of the prostate. Am J Surg Pathol. 1988;12:619–33.
Costello LC, Franklin RB. A comprehensive review of the role of zinc in normal prostate function and metabolism; and its implications in prostate cancer. Arch Biochem Biophys. 2016;611:100–12.
Costello LC, Feng P, Milon B, Tan M, Franklin RB. Role of zinc in the pathogenesis and treatment of prostate cancer: critical issues to resolve. Prostate Cancer Prostatic Dis. 2004;7:111–7.
Costello LC, Franklin RB. Cytotoxic/tumor suppressor role of zinc for the treatment of cancer: an enigma and an opportunity. Expert Rev Anticancer Ther. 2012;12:121–8.
Sandhu S, Moore CM, Chiong E, Beltran H, Bristow RG, Williams SG. Prostate cancer. Lancet. 2021;398:1075–90.
Jette NR, Radhamani S, Ye R, Yu Y, Arthur G, Goutam S, et al. ATM-deficient lung, prostate and pancreatic cancer cells are acutely sensitive to the combination of olaparib and the ATR inhibitor AZD6738. Genome Instab Dis. 2020;1:197–205.
Zhao Y, Hu X, Yu H, Sun H, Zhang L, Shao C. The FTO mediated N6-methyladenosine modification of DDIT4 regulation with tumorigenesis and metastasis in prostate cancer. Res (Wash D C). 2024;7:0313.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Muhamed PK, Vadstrup S. [Zinc is the most important trace element]. Ugeskr Laeger. 2014;176:V11120654.
Takatani-Nakase T. Zinc transporters and the progression of breast cancers. Biol Pharm Bull. 2018;41:1517–22.
Yu Z, Yu Z, Chen Z, Yang L, Ma M, Lu S, et al. Zinc chelator TPEN induces pancreatic cancer cell death through causing oxidative stress and inhibiting cell autophagy. J Cell Physiol. 2019;234:20648–61.
Ninsontia C, Phiboonchaiyanan PP, Kiratipaiboon C, Chanvorachote P. Zinc suppresses stem cell properties of lung cancer cells through protein kinase C-mediated beta-catenin degradation. Am J Physiol Cell Physiol. 2017;312:C487–99.
Hosui A, Kimura E, Abe S, Tanimoto T, Onishi K, Kusumoto Y, et al. Long-term zinc supplementation improves liver function and decreases the risk of developing hepatocellular carcinoma. Nutrients. 2018;10:1955.
Kim S, Jung Y, Kim D, Koh H, Chung J. Extracellular zinc activates p70 S6 kinase through the phosphatidylinositol 3-kinase signaling pathway. J Biol Chem. 2000;275:25979–84.
Eom SJ, Kim EY, Lee JE, Kang HJ, Shim J, Kim SU, et al. Zn(2+) induces stimulation of the c-Jun N-terminal kinase signaling pathway through phosphoinositide 3-Kinase. Mol Pharm. 2001;59:981–6.
Tang X, Shay NF. Zinc has an insulin-like effect on glucose transport mediated by phosphoinositol-3-kinase and Akt in 3T3-L1 fibroblasts and adipocytes. J Nutr. 2001;131:1414–20.
Pandey NR, Vardatsikos G, Mehdi MZ, Srivastava AK. Cell-type-specific roles of IGF-1R and EGFR in mediating Zn2+-induced ERK1/2 and PKB phosphorylation. J Biol Inorg Chem. 2010;15:399–407.
Vardatsikos G, Pandey NR, Srivastava AK. Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem. 2013;120:8–17.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74.
Parikh C, Janakiraman V, Wu WI, Foo CK, Kljavin NM, Chaudhuri S, et al. Disruption of PH-kinase ___domain interactions leads to oncogenic activation of AKT in human cancers. Proc Natl Acad Sci USA. 2012;109:19368–73.
Alessi DR, Andjelkovic M, Caudwell B, Cron P, Morrice N, Cohen P, et al. Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 1996;15:6541–51.
Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter GF, Holmes AB, et al. Protein kinase B kinases that mediate phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–4.
Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–101.
Calleja V, Alcor D, Laguerre M, Park J, Vojnovic B, Hemmings BA, et al. Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo. PLoS Biol. 2007;5:e95.
Zaichick V, Sviridova TV, Zaichick SV. Zinc in the human prostate gland: normal, hyperplastic and cancerous. Int Urol Nephrol. 1997;29:565–74.
Banas A, Kwiatek WM, Banas K, Gajda M, Pawlicki B, Cichocki T. Correlation of concentrations of selected trace elements with Gleason grade of prostate tissues. J Biol Inorg Chem. 2010;15:1147–55.
Kolenko V, Teper E, Kutikov A, Uzzo R. Zinc and zinc transporters in prostate carcinogenesis. Nat Rev Urol. 2013;10:219–26.
Anson KJ, Corbet GA, Palmer AE. Zn(2+) influx activates ERK and Akt signaling pathways. Proc Natl Acad Sci USA. 2021;118:e2015786118.
Bellacosa A, Chan TO, Ahmed NN, Datta K, Malstrom S, Stokoe D, et al. Akt activation by growth factors is a multiple-step process: the role of the PH ___domain. Oncogene. 1998;17:313–25.
Abramson J, Adler J, Dunger J, Evans R, Green T, Pritzel A, et al. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500.
Milburn CC, Deak M, Kelly SM, Price NC, Alessi DR, Van Aalten DM. Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology ___domain of protein kinase B induces a conformational change. Biochem J. 2003;375:531–8.
Yang WL, Wang J, Chan CH, Lee SW, Campos AD, Lamothe B, et al. The E3 ligase TRAF6 regulates Akt ubiquitination and activation. Science. 2009;325:1134–8.
James SR, Downes CP, Gigg R, Grove SJ, Holmes AB, Alessi DR. Specific binding of the Akt-1 protein kinase to phosphatidylinositol 3,4,5-trisphosphate without subsequent activation. Biochem J. 1996;315:709–13.
Rehan M, Beg MA, Parveen S, Damanhouri GA, Zaher GF. Computational insights into the inhibitory mechanism of human AKT1 by an orally active inhibitor, MK-2206. PLoS One. 2014;9:e109705.
Li D, Stovall DB, Wang W, Sui G. Advances of zinc signaling studies in prostate cancer. Int J Mol Sci. 2020;21:667.
Stefanidou M, Maravelias C, Dona A, Spiliopoulou C. Zinc: a multipurpose trace element. Arch Toxicol. 2006;80:1–9.
Sapota A, Darago A, Taczalski J, Kilanowicz A. Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions. Biometals. 2009;22:1041–9.
Epstein MM, Kasperzyk JL, Andren O, Giovannucci EL, Wolk A, Hakansson N, et al. Dietary zinc and prostate cancer survival in a Swedish cohort. Am J Clin Nutr. 2011;93:586–93.
Gallus S, Foschi R, Negri E, Talamini R, Franceschi S, Montella M, et al. Dietary zinc and prostate cancer risk: a case-control study from Italy. Eur Urol. 2007;52:1052–6.
Zhang Y, Song M, Mucci LA, Giovannucci EL. Zinc supplement use and risk of aggressive prostate cancer: a 30-year follow-up study. Eur J Epidemiol. 2022;37:1251–60.
Guo J, Dai X, Laurent B, Zheng N, Gan W, Zhang J, et al. AKT methylation by SETDB1 promotes AKT kinase activity and oncogenic functions. Nat Cell Biol. 2019;21:226–37.
Wang G, Long J, Gao Y, Zhang W, Han F, Xu C, et al. SETDB1-mediated methylation of Akt promotes its K63-linked ubiquitination and activation leading to tumorigenesis. Nat Cell Biol. 2019;21:214–25.
Guo J, Cheng J, Zheng N, Zhang X, Dai X, Zhang L, et al. Copper promotes tumorigenesis by activating the PDK1-AKT oncogenic pathway in a copper transporter 1 dependent manner. Adv Sci (Weinh). 2021;8:e2004303.
Drost J, Karthaus WR, Gao D, Driehuis E, Sawyers CL, Chen Y, et al. Organoid culture systems for prostate epithelial and cancer tissue. Nat Protoc. 2016;11:347–58.
Lukacs RU, Goldstein AS, Lawson DA, Cheng D, Witte ON. Isolation, cultivation and characterization of adult murine prostate stem cells. Nat Protoc. 2010;5:702–13.
Chen M, Wang K, Han Y, Yan S, Yuan H, Liu Q, et al. Identification of XAF1 as an endogenous AKT inhibitor. Cell Rep. 2023;42:112690.
Acknowledgements
We thank Dr. Dangsheng Li for kindly providing great helpful suggestions.
Funding
This work was supported by the National Key Research and Development Program of China (grants 2020YFA0803203, 2019YFA0802102), grants from National Natural Science Foundation of China (81925029, 81790253, 82230098, 32221002), the Strategic Priority Research Program of the Chinese Academy of Sciences (XDB19020203 and XDB37010303), and the CAS project for young scientists in basic research (YSBR-014) and Shanghai Municipal Science and Technology Major Project.
Author information
Authors and Affiliations
Contributions
DG conceived the study. DG, JQ, and YC supervised the study with input from HZ. Kangjunjie W, MC, and SY performed the bench experiments and analyzed the data with assistance from YH, HY, LL, Kaihua W, FL, Qianqian L, Dakui L. Dayun L. Qiuli L and JJ collected and provided the clinical prostate samples. DG and Kangjunjie W wrote the manuscript. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, K., Chen, M., Yan, S. et al. Zinc ions activate AKT and promote prostate cancer cell proliferation via disrupting AKT intramolecular interaction. Oncogene 44, 8–18 (2025). https://doi.org/10.1038/s41388-024-03195-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-03195-x