Abstract
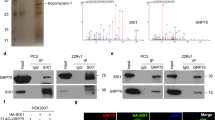
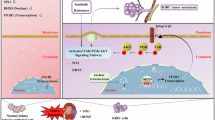
Sine oculis homeobox homolog 1 (SIX1) is a new identified cancer driver in the development of prostate cancer (PC). However, the upstream regulatory mechanisms for SIX1 reactivation in cancer remains elusive. Here, we found that Ku70 robustly interacts with SIX1 in the nucleus of PC cells. The HD ___domain of SIX1 and the DBD ___domain of Ku70 are required for formation of Ku70-SIX1 complex. 20 groups of hydrogen bonds were identified in this complex by molecular dynamics simulation. Depletion of Ku70/SIX1 notably abrogates the proliferation and migration of PC. Further studies revealed that SIX1 is recruited to the promoter region on glutamate-pyruvate transaminase 2 (GPT2). Ku70 enhances the SIX1-mediated transcriptional activation on GPT2, thereby facilitating the generation of alpha-ketoglutarate (α-KG). In addition, formation of the Ku70-SIX1 complex promotes GPT2-dependent cell proliferation and migration in PC. Moreover, the expression of GPT2 is upregulated and strongly correlated with the expression of Ku70/SIX1 in PC tissues. In summary, our findings not only provide insight into the mechanistic interactions between Ku70 and SIX1, but also highlight the significance of the Ku70-SIX1-GPT2 axis for α-KG metabolism and PC carcinogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All datasets and materials presented in this study are available from the corresponding author on reasonable request.
References
Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clinicians. 2024;74:229–63.
Li Z, Alyamani M, Li J, Rogacki K, Abazeed M, Upadhyay SK, et al. Redirecting abiraterone metabolism to fine-tune prostate cancer anti-androgen therapy. Nature. 2016;533:547–51.
Sullivan WJ, Mullen PJ, Schmid EW, Flores A, Momcilovic M, Sharpley MS, et al. Extracellular Matrix Remodeling Regulates Glucose Metabolism through TXNIP Destabilization. Cell. 2018;175:117–32.e21.
Vriens K, Christen S, Parik S, Broekaert D, Yoshinaga K, Talebi A, et al. Evidence for an alternative fatty acid desaturation pathway increasing cancer plasticity. Nature. 2019;566:403–6.
Mossmann D, Müller C, Park S, Ryback B, Colombi M, Ritter N, et al. Arginine reprograms metabolism in liver cancer via RBM39. Cell. 2023;186:5068–83.e23.
Ricci L, Stanley FU, Eberhart T, Mainini F, Sumpton D, Cardaci S. Pyruvate transamination and NAD biosynthesis enable proliferation of succinate dehydrogenase-deficient cells by supporting aerobic glycolysis. Cell Death Dis. 2023;14:403.
Li N, Xu X, Liu D, Gao J, Gao Y, Wu X, et al. The delta subunit of the GABA(A) receptor is necessary for the GPT2-promoted breast cancer metastasis. Theranostics. 2023;13:1355–69.
Mitra D, Vega-Rubin-de-Celis S, Royla N, Bernhardt S, Wilhelm H, Tarade N, et al. Abrogating GPT2 in triple-negative breast cancer inhibits tumor growth and promotes autophagy. Int J Cancer. 2021;148:1993–2009.
Li J, Qin Z, Li Y, Huang B, Xiao Q, Chen P, et al. Phosphorylation of IDH1 Facilitates Progestin Resistance in Endometrial Cancer. Adv Sci. 2024;11:e2310208.
Zarei M, Hajihassani O, Hue JJ, Loftus AW, Graor HJ, Nakazzi F, et al. IDH1 inhibition potentiates chemotherapy efficacy in pancreatic cancer. Cancer Res. 2024;84:3072–85.
Li JJ, Yu T, Zeng P, Tian J, Liu P, Qiao S, et al. Wild-type IDH2 is a therapeutic target for triple-negative breast cancer. Nat Commun. 2024;15:3445.
Spitz F, Demignon J, Porteu A, Kahn A, Concordet JP, Daegelen D, et al. Expression of myogenin during embryogenesis is controlled by Six/sine oculis homeoproteins through a conserved MEF3 binding site. Proc Natl Acad Sci USA. 1998;95:14220–5.
Li X, Oghi KA, Zhang J, Krones A, Bush KT, Glass CK, et al. Eya protein phosphatase activity regulates Six1-Dach-Eya transcriptional effects in mammalian organogenesis. Nature. 2003;426:247–54.
Li L, Liang Y, Kang L, Liu Y, Gao S, Chen S, et al. Transcriptional Regulation of the Warburg Effect in Cancer by SIX1. Cancer Cell. 2018;33:368–85.e7.
Liu Y, Kong WY, Yu CF, Shao ZL, Lei QC, Deng YF, et al. SNS-023 sensitizes hepatocellular carcinoma to sorafenib by inducing degradation of cancer drivers SIX1 and RPS16. Acta Pharmacologica Sin. 2023;44:853–64.
Liao Y, Liu Y, Shao Z, Xia X, Deng Y, Cai J, et al. A new role of GRP75-USP1-SIX1 protein complex in driving prostate cancer progression and castration resistance. Oncogene. 2021;40:4291–306.
Chu Y, Jiang M, Wu N, Xu B, Li W, Liu H, et al. O-GlcNAcylation of SIX1 enhances its stability and promotes Hepatocellular Carcinoma Proliferation. Theranostics. 2020;10:9830–42.
Camolotto SA, Belova VK, Torre-Healy L, Vahrenkamp JM, Berrett KC, Conway H, et al. Reciprocal regulation of pancreatic ductal adenocarcinoma growth and molecular subtype by HNF4α and SIX1/4. Gut. 2021;70:900–14.
Zhu Z, Rong Z, Luo Z, Yu Z, Zhang J, Qiu Z, et al. Circular RNA circNHSL1 promotes gastric cancer progression through the miR-1306-3p/SIX1/vimentin axis. Mol Cancer. 2019;18:126.
Liao Y, Sun W, Shao Z, Liu Y, Zhong X, Deng Y, et al. A SIX1 degradation inducer blocks excessive proliferation of prostate cancer. Int J Biol Sci. 2022;18:2439–51.
Al-Ubaidi FL, Schultz N, Loseva O, Egevad L, Granfors T, Helleday T. Castration therapy results in decreased Ku70 levels in prostate cancer. Clin Cancer Res. 2013;19:1547–56.
Jin S, Weaver DT. Double-strand break repair by Ku70 requires heterodimerization with Ku80 and DNA binding functions. EMBO J. 1997;16:6874–85.
Yang Q, Xu J, Gu J, Shi H, Zhang J, Zhang J, et al. Extracellular Vesicles in Cancer Drug Resistance: Roles, Mechanisms, and Implications. Adv Sci. 2022;9:e2201609.
Wang X, Liu R, Zhu W, Chu H, Yu H, Wei P, et al. UDP-glucose accelerates SNAI1 mRNA decay and impairs lung cancer metastasis. Nature. 2019;571:127–31.
Kim M, Gwak J, Hwang S, Yang S, Jeong SM. Mitochondrial GPT2 plays a pivotal role in metabolic adaptation to the perturbation of mitochondrial glutamine metabolism. Oncogene. 2019;38:4729–38.
Wang CA, Jedlicka P, Patrick AN, Micalizzi DS, Lemmer KC, Deitsch E, et al. SIX1 induces lymphangiogenesis and metastasis via upregulation of VEGF-C in mouse models of breast cancer. J Clin Investig. 2012;122:1895–906.
Iwanaga R, Wang CA, Micalizzi DS, Harrell JC, Jedlicka P, Sartorius CA, et al. Expression of Six1 in luminal breast cancers predicts poor prognosis and promotes increases in tumor initiating cells by activation of extracellular signal-regulated kinase and transforming growth factor-beta signaling pathways. Breast Cancer Res. 2012;14:R100.
Wu K, Li Z, Cai S, Tian L, Chen K, Wang J, et al. EYA1 phosphatase function is essential to drive breast cancer cell proliferation through cyclin D1. Cancer Res. 2013;73:4488–99.
Hsu JY, Danis EP, Nance S, O’Brien JH, Gustafson AL, Wessells VM, et al. SIX1 reprograms myogenic transcription factors to maintain the rhabdomyosarcoma undifferentiated state. Cell Rep. 2022;38:110323.
Liu W, Gao M, Li L, Chen Y, Fan H, Cai Q, et al. Homeoprotein SIX1 compromises antitumor immunity through TGF-β-mediated regulation of collagens. Cell Mol Immunol. 2021;18:2660–72.
Park SJ, Ciccone SL, Freie B, Kurimasa A, Chen DJ, Li GC, et al. A positive role for the Ku complex in DNA replication following strand break damage in mammals. J Biol Chem. 2004;279:6046–55.
Rivera-Calzada A, Spagnolo L, Pearl LH, Llorca O. Structural model of full-length human Ku70-Ku80 heterodimer and its recognition of DNA and DNA-PKcs. EMBO Rep. 2007;8:56–62.
Li Q, Yao H, Wang Y, Wu Y, Thorne RF, Zhu Y, et al. circPRKAA1 activates a Ku80/Ku70/SREBP-1 axis driving de novo fatty acid synthesis in cancer cells. Cell Rep. 2022;41:111707.
Yu Y, Liu T, Yu G, Wang H, Du Z, Chen Y, et al. PRDM15 interacts with DNA-PK-Ku complex to promote radioresistance in rectal cancer by facilitating DNA damage repair. Cell Death Dis. 2022;13:978.
Wang B, Xie M, Li R, Owonikoko TK, Ramalingam SS, Khuri FR, et al. Role of Ku70 in deubiquitination of Mcl-1 and suppression of apoptosis. Cell Death Differ. 2014;21:1160–9.
Shu Y, Jin X, Ji M, Zhang Z, Wang X, Liang H, et al. Ku70 Binding to YAP Alters PARP1 Ubiquitination to Regulate Genome Stability and Tumorigenesis. Cancer Res. 2024;84:2836–55.
Acknowledgements
This work was supported by National Natural Science Foundation of China (82072810, 82373327), Plan on enhancing scientific research in Guangzhou Medical University, Cultivation Program of National Natural Science Foundation for Distinguished Young Scholars of Guangzhou Medical University (JP2022002), Innovation team of general Universities in Guangdong Province (2022KCXTD021), Discipline Construction Funds of Guangzhou Medical University (JCXKJS2022A06), Guangdong Basic and Applied Basic Research Foundation (2021A1515111087), Medical research project of Foshan Health Bureau (20220479).
Author information
Authors and Affiliations
Contributions
HBH, XFZ, and SSY contributed equally to this work. YNL, GXC, and HBH conceived the ideas and designed the experiments. HBH, XFZ, SSY, WSS, JC, EYP, YJX, XYH, MFT, YTL, YY, YFD, QL, ZLS, XHX, and GXC performed the experiments. YNL and HBH wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. Informed consent was obtained from all the subjects. All animal experiments were approved by the institutional animal care and use committees of Guangzhou Medical University(GY2022-137). All human subjects were performed with the approval of the Medical Ethics Committee of the First People’s Hospital of Foshan (ethics approval number: L [2024] No. 3).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Huang, H., Zhuang, X., Yin, S. et al. The Ku70-SIX1-GPT2 axis regulates alpha-ketoglutarate metabolism to drive progression of prostate cancer. Oncogene 44, 92–104 (2025). https://doi.org/10.1038/s41388-024-03209-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-03209-8