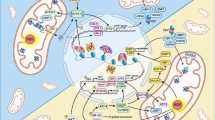
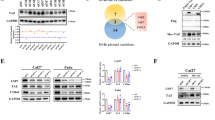
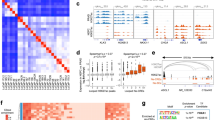
Abstract
The mitochondrial unfolded protein response (UPRmt) maintains mitochondrial quality control and proteostasis under stress conditions. However, the role of UPRmt in aggressive and resistant prostate cancer is not clearly defined. We show that castration-resistant neuroendocrine prostate cancer (CRPC-NE) harbored highly dysfunctional oxidative phosphorylation (OXPHOS) Complexes. However, biochemical and protein analyses of CRPC-NE tumors showed upregulation of nuclear-encoded OXPHOS proteins and UPRmt in this lethal subset of prostate cancer suggestive of compensatory upregulation of stress signaling. Genetic deletion and pharmacological inhibition of the main chaperone of UPRmt heat shock protein 60 (HSP60) reduced neuroendocrine prostate cancer (NEPC) growth in vivo as well as reverted NEPC cells to a more epithelial-like state. HSP60-dependent aggressive NEPC phenotypes was associated with upregulation of β-catenin signaling both in cancer cells and in vivo tumors. HSP60 expression rendered enrichment of aggressive prostate cancer signatures and metastatic potential were inhibited upon suppression of UPRmt. We discovered that UPRmt promoted OXPHOS functions including mitochondrial bioenergetics in CRPC-NE via regulation of β-catenin signaling. Mitochondrial biogenesis facilitated cisplatin resistance and inhibition of UPRmt resensitizes CRPC-NE cells to cisplatin. Together, our findings demonstrated that UPRmt promotes mitochondrial health via upregulating β-catenin signaling and UPRmt represents viable therapeutic target for NEPC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data associated with this study are present in the paper and/or the Supplementary informations.
References
Siegel RL, Giaquinto AN, Jemal A. Cancer statistics, 2024. CA Cancer J Clin. 2024;74:12–49.
Culig Z. Targeting the androgen receptor in prostate cancer. Expert Opin Pharmacother. 2014;15:1427–37.
Beltran H, Hruszkewycz A, Scher HI, Hildesheim J, Isaacs J, Yu EY, et al. The role of lineage plasticity in prostate cancer therapy resistance. Clin Cancer Res. 2019;25:6916–24.
Bluemn EG, Coleman IM, Lucas JM, Coleman RT, Hernandez-Lopez S, Tharakan R, et al. Androgen receptor pathway-independent prostate cancer is sustained through FGF signaling. Cancer Cell. 2017;32:474–89.e476.
Ku SY, Rosario S, Wang Y, Mu P, Seshadri M, Goodrich ZW, et al. Rb1 and Trp53 cooperate to suppress prostate cancer lineage plasticity, metastasis, and antiandrogen resistance. Science. 2017;355:78–83.
Spetsieris N, Boukovala M, Patsakis G, Alafis I, Efstathiou E. Neuroendocrine and aggressive-variant prostate cancer. Cancers. 2020;12:3792.
Aparicio AM, Harzstark AL, Corn PG, Wen S, Araujo JC, Tu SM, et al. Platinum-based chemotherapy for variant castrate-resistant prostate cancer. Clin Cancer Res. 2013;19:3621–30.
Rattan SIS, Kyriazis M. The science of hormesis in health and longevity. Elsevier/Academic Press: London, United Kingdom, 2019.
O’Malley J, Kumar R, Inigo J, Yadava N, Chandra D. Mitochondrial stress response and cancer. Trends Cancer. 2020;6:688–701.
Fiorese CJ, Schulz AM, Lin YF, Rosin N, Pellegrino MW, Haynes CM. The transcription factor ATF5 mediates a mammalian mitochondrial UPR. Curr Biol. 2016;26:2037–43.
Mu P, Zhang Z, Benelli M, Karthaus WR, Hoover E, Chen CC, et al. SOX2 promotes lineage plasticity and antiandrogen resistance in TP53- and RB1-deficient prostate cancer. Science. 2017;355:84–88.
Cappello F, Conway de Macario E, Marasa L, Zummo G, Macario AJ. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol Ther. 2008;7:801–9.
Zhou C, Sun H, Zheng C, Gao J, Fu Q, Hu N, et al. Oncogenic HSP60 regulates mitochondrial oxidative phosphorylation to support Erk1/2 activation during pancreatic cancer cell growth. Cell Death Dis. 2018;9:161.
Kumar R, Chaudhary AK, Woytash J, Inigo JR, Gokhale AA, Bshara W, et al. A mitochondrial unfolded protein response inhibitor suppresses prostate cancer growth in mice via HSP60. J Clin Invest. 2022;132:e149906.
Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–66.
Woytash J, Inigo JR, Chandra D. Assessing oligomerization status of mitochondrial OXPHOS Complexes via blue native page. Methods Mol Biol. 2022;2413:55–62.
Gingrich JR, Barrios RJ, Foster BA, Greenberg NM. Pathologic progression of autochthonous prostate cancer in the TRAMP model. Prostate Cancer Prostatic Dis. 1999;2:70–75.
Letts JA, Sazanov LA. Clarifying the supercomplex: the higher-order organization of the mitochondrial electron transport chain. Nat Struct Mol Biol. 2017;24:800–8.
Wenz T. Regulation of mitochondrial biogenesis and PGC-1alpha under cellular stress. Mitochondrion. 2013;13:134–42.
Tan HL, Sood A, Rahimi HA, Wang W, Gupta N, Hicks J, et al. Rb loss is characteristic of prostatic small cell neuroendocrine carcinoma. Clin Cancer Res. 2014;20:890–903.
Ciarlo M, Benelli R, Barbieri O, Minghelli S, Barboro P, Balbi C, et al. Regulation of neuroendocrine differentiation by AKT/hnRNPK/AR/beta-catenin signaling in prostate cancer cells. Int J Cancer. 2012;131:582–90.
Moparthi L, Pizzolato G, Koch S. Wnt activator FOXB2 drives the neuroendocrine differentiation of prostate cancer. Proc Natl Acad Sci USA. 2019;116:22189–95.
Unno K, Chalmers ZR, Pamarthy S, Vatapalli R, Rodriguez Y, Lysy B, et al. Activated ALK cooperates with N-Myc via Wnt/beta-catenin signaling to induce neuroendocrine prostate cancer. Cancer Res. 2021;81:2157–70.
Hailesellasse Sene K, Porter CJ, Palidwor G, Perez-Iratxeta C, Muro EM, et al. Gene function in early mouse embryonic stem cell differentiation. BMC Genom. 2007;8:85.
Vergara D, Stanca E, Guerra F, Priore P, Gaballo A, Franck J, et al. Beta-catenin knockdown affects mitochondrial biogenesis and lipid metabolism in breast cancer cells. Front Physiol. 2017;8:544.
Ma Y, Ma M, Sun J, Li W, Li Y, Guo X, et al. CHIR-99021 regulates mitochondrial remodelling via beta-catenin signalling and miRNA expression during endodermal differentiation. J Cell Sci. 2019;132:jcs229948.
Akamatsu S, Inoue T, Ogawa O, Gleave ME. Clinical and molecular features of treatment-related neuroendocrine prostate cancer. Int J Urol. 2018;25:345–51.
Flechon A, Pouessel D, Ferlay C, Perol D, Beuzeboc P, Gravis G, et al. Phase II study of carboplatin and etoposide in patients with anaplastic progressive metastatic castration-resistant prostate cancer (mCRPC) with or without neuroendocrine differentiation: results of the French Genito-Urinary Tumor Group (GETUG) P01 trial. Ann Oncol. 2011;22:2476–81.
Waseem M, Bhardwaj M, Tabassum H, Raisuddin S, Parvez S. Cisplatin hepatotoxicity mediated by mitochondrial stress. Drug Chem Toxicol. 2015;38:452–9.
Yang Z, Schumaker LM, Egorin MJ, Zuhowski EG, Guo Z, Cullen KJ. Cisplatin preferentially binds mitochondrial DNA and voltage-dependent anion channel protein in the mitochondrial membrane of head and neck squamous cell carcinoma: possible role in apoptosis. Clin Cancer Res. 2006;12:5817–25.
Podratz JL, Knight AM, Ta LE, Staff NP, Gass JM, Genelin K, et al. Cisplatin induced mitochondrial DNA damage in dorsal root ganglion neurons. Neurobiol Dis. 2011;41:661–8.
Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, et al. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS ONE. 2013;8:e81162.
Tang Y, Yang Y, Luo J, Liu S, Zhan Y, Zang H, et al. Overexpression of HSP10 correlates with HSP60 and Mcl-1 levels and predicts poor prognosis in non-small cell lung cancer patients. Cancer Biomark. 2021;30:85–94.
Rappa F, Pitruzzella A, Marino Gammazza A, Barone R, Mocciaro E, Tomasello G, et al. Quantitative patterns of Hsps in tubular adenoma compared with normal and tumor tissues reveal the value of Hsp10 and Hsp60 in early diagnosis of large bowel cancer. Cell Stress Chaperones. 2016;21:927–33.
Chen FM, Huang LJ, Ou-Yang F, Kan JY, Kao LC, Hou MF. Activation of mitochondrial unfolded protein response is associated with Her2-overexpression breast cancer. Breast Cancer Res Treat. 2020;183:61–70.
Huang YH, Lin KH, Yu JS, Wu TJ, Lee WC, Chao CC, et al. Targeting HSP60 by subcutaneous injections of jetPEI/HSP60-shRNA destabilizes cytoplasmic survivin and inhibits hepatocellular carcinoma growth. Mol Carcinog. 2018;57:1087–101.
Tang H, Li J, Liu X, Wang G, Luo M, Deng H. Down-regulation of HSP60 Suppresses the Proliferation of Glioblastoma Cells via the ROS/AMPK/mTOR Pathway. Sci Rep. 2016;6:28388.
Li XS, Xu Q, Fu XY, Luo WS. Heat shock protein 60 overexpression is associated with the progression and prognosis in gastric cancer. PLoS ONE. 2014;9:e107507.
Campanella C, Rappa F, Sciume C, Marino Gammazza A, Barone R, Bucchieri F, et al. Heat shock protein 60 levels in tissue and circulating exosomes in human large bowel cancer before and after ablative surgery. Cancer. 2015;121:3230–9.
He Y, Wu Y, Mou Z, Li W, Zou L, Fu T, et al. Proteomics-based identification of HSP60 as a tumor-associated antigen in colorectal cancer. Proteom Clin Appl. 2007;1:336–42.
Wu X, Guo J, Chen Y, Liu X, Yang G, Wu Y, et al. The 60-kDa heat shock protein regulates energy rearrangement and protein synthesis to promote proliferation of multiple myeloma cells. Br J Haematol. 2020;190:741–52.
Hwang YJ, Lee SP, Kim SY, Choi YH, Kim MJ, Lee CH, et al. Expression of heat shock protein 60 kDa is upregulated in cervical cancer. Yonsei Med J. 2009;50:399–406.
Guo J, Li X, Zhang W, Chen Y, Zhu S, Chen L, et al. HSP60-regulated mitochondrial proteostasis and protein translation promote tumor growth of ovarian cancer. Sci Rep. 2019;9:12628.
Rusthoven CG, Carlson JA, Waxweiler TV, Yeh N, Raben D, Flaig TW, et al. The prognostic significance of Gleason scores in metastatic prostate cancer. Urol Oncol. 2014;32:707–13.
Gleason DF. Classification of prostatic carcinomas. Cancer Chemother Rep. 1966;50:125–8.
Ross RW, Xie W, Regan MM, Pomerantz M, Nakabayashi M, Daskivich TJ, et al. Efficacy of androgen deprivation therapy (ADT) in patients with advanced prostate cancer: association between Gleason score, prostate-specific antigen level, and prior ADT exposure with duration of ADT effect. Cancer. 2008;112:1247–53.
de la Taille A, Rubin MA, Chen MW, Vacherot F, de Medina SG, Burchardt M, et al. Beta-catenin-related anomalies in apoptosis-resistant and hormone-refractory prostate cancer cells. Clin Cancer Res. 2003;9:1801–7.
Chen G, Shukeir N, Potti A, Sircar K, Aprikian A, Goltzman D, et al. Up-regulation of Wnt-1 and beta-catenin production in patients with advanced metastatic prostate carcinoma: potential pathogenetic and prognostic implications. Cancer. 2004;101:1345–56.
Wan X, Liu J, Lu JF, Tzelepi V, Yang J, Starbuck MW, et al. Activation of beta-catenin signaling in androgen receptor-negative prostate cancer cells. Clin Cancer Res. 2012;18:726–36.
Lee E, Ha S, Logan SK. Divergent Androgen receptor and beta-catenin signaling in prostate cancer cells. PLoS ONE. 2015;10:e0141589.
Yan FQ, Wang JQ, Tsai YP, Wu KJ. HSP60 overexpression increases the protein levels of the p110alpha subunit of phosphoinositide 3-kinase and c-Myc. Clin Exp Pharm Physiol. 2015;42:1092–7.
Tsai YP, Yang MH, Huang CH, Chang SY, Chen PM, Liu CJ, et al. Interaction between HSP60 and beta-catenin promotes metastasis. Carcinogenesis. 2009;30:1049–57.
Brown-Clay JD, Shenoy DN, Timofeeva O, Kallakury BV, Nandi AK, Banerjee PP. PBK/TOPK enhances aggressive phenotype in prostate cancer via beta-catenin-TCF/LEF-mediated matrix metalloproteinases production and invasion. Oncotarget. 2015;6:15594–609.
Li X, Xu Y, Chen Y, Chen S, Jia X, Sun T, et al. SOX2 promotes tumor metastasis by stimulating epithelial-to-mesenchymal transition via regulation of WNT/beta-catenin signal network. Cancer Lett. 2013;336:379–89.
Yu X, Wang Y, Jiang M, Bierie B, Roy-Burman P, Shen MM, et al. Activation of beta-Catenin in mouse prostate causes HGPIN and continuous prostate growth after castration. Prostate. 2009;69:249–62.
Francis JC, Thomsen MK, Taketo MM, Swain A. beta-catenin is required for prostate development and cooperates with Pten loss to drive invasive carcinoma. PLoS Genet. 2013;9:e1003180.
Costa R, Peruzzo R, Bachmann M, Monta GD, Vicario M, Santinon G, et al. Impaired mitochondrial ATP production downregulates Wnt signaling via ER stress induction. Cell Rep. 2019;28:1949–60.e1946.
Mezhybovska M, Yudina Y, Abhyankar A, Sjolander A. Beta-catenin is involved in alterations in mitochondrial activity in non-transformed intestinal epithelial and colon cancer cells. Br J Cancer. 2009;101:1596–605.
Yoon JC, Ng A, Kim BH, Bianco A, Xavier RJ, Elledge SJ. Wnt signaling regulates mitochondrial physiology and insulin sensitivity. Genes Dev. 2010;24:1507–18.
Bernkopf DB, Jalal K, Bruckner M, Knaup KX, Gentzel M, Schambony A, et al. Pgam5 released from damaged mitochondria induces mitochondrial biogenesis via Wnt signaling. J Cell Biol. 2018;217:1383–94.
De Luca A, Fiorillo M, Peiris-Pages M, Ozsvari B, Smith DL, Sanchez-Alvarez R, et al. Mitochondrial biogenesis is required for the anchorage-independent survival and propagation of stem-like cancer cells. Oncotarget. 2015;6:14777–95.
Vellinga TT, Borovski T, de Boer VC, Fatrai S, van Schelven S, Trumpi K, et al. SIRT1/PGC1alpha-dependent increase in oxidative phosphorylation supports chemotherapy resistance of colon cancer. Clin Cancer Res. 2015;21:2870–9.
Salem AF, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP. Mitochondrial biogenesis in epithelial cancer cells promotes breast cancer tumor growth and confers autophagy resistance. Cell Cycle. 2012;11:4174–80.
Molina JR, Sun Y, Protopopova M, Gera S, Bandi M, Bristow C, et al. An inhibitor of oxidative phosphorylation exploits cancer vulnerability. Nat Med. 2018;24:1036–46.
Funding
This work was supported by the NCI of the NIH under Award Number R01-CA160685 and R01CA246437 (to D. Chandra), the American Cancer Society under award number MBG-21-048-01-MBG and RSG-12-214-01 – CCG (to D. Chandra), Roswell Park Alliance Foundation (to DC), and in part by the NCI Center Support Grant P30-CA016056 to the Roswell Park Comprehensive Cancer Center that supports the Translational Imaging, Pathology Network, Flow and Image Cytometry, Bioinformatics, Biostatistics, Genomic, Animal housing facility Shared Resources and the Onsite Supply Center. We thank Drs. David Goodrich, Dean G Tang, Barbara Foster, and Raghu Gogada for their technical support and reagents.
Author information
Authors and Affiliations
Contributions
DC, JAW, and RK designed experiments. JAW, RK, AKC, CD, AW, NY, MB, JRI, and JW performed experiments. JS helped with analysis of MR imaging and PB provided HSPD1 mouse. JAW performed statistical and bioinformatic analysis. DC, JAW, and RK analyzed the data. DC and JAW wrote the paper. DC conceived the idea and supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
The authors confirm that all methods were performed in accordance with the relevant guidelines and regulations. For all mouse experiments (including all GEMM and tumor xenograft experiments), approval has been obtained from the Institutional Animal Care and Use Committee at Roswell Park Comprehensive Cancer Center (IACUC Approval # 1306 M). This work does not involve human research.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Woytash, J.A., Kumar, R., Chaudhary, A.K. et al. Mitochondrial unfolded protein response-dependent β-catenin signaling promotes neuroendocrine prostate cancer. Oncogene 44, 820–834 (2025). https://doi.org/10.1038/s41388-024-03261-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-024-03261-4
This article is cited by
-
Turning down the heat in NEPC
Nature Reviews Urology (2025)