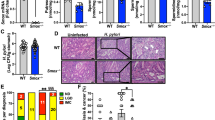
Abstract
DOP1 leucine zipper-like protein A (DOPEY1), a member of the DOPEY family, is mainly localized in the Golgi apparatus, endosomes, and cytoplasmic compartments within cells. The involvement of DOPEY1 in H. pylori infection-induced carcinogenesis has remained unresolved. Here, we report that DOPEY1 is upregulated in GC tissues compared to adjacent normal tissues, correlating with poor prognosis. Mechanistically, H. pylori infection increases DOPEY1 expression and promotes p53 degradation through a CagA-dependent pathway. Using the String database and liquid chromatography-mass spectrometry, we identified DOPEY1-interacting proteins, confirming through co-immunoprecipitation that DOPEY1 interacts with USP7 and TRIP12. H. pylori infection enhances the expression of DOPEY1, USP7, and TRIP12, leading to p53 degradation, which is reversed by DOPEY1 silencing. Moreover, USP7 overexpression rescues p53 degradation in DOPEY1-silenced cells. Functionally, DOPEY1 knockdown reduces GC cell proliferation and suppresses tumor growth in mouse models. Immunohistochemistry analysis further reveals a link between DOPEY1, USP7, and TRIP12 expression, H. pylori infection, and GC progression. These findings demonstrate that H. pylori-induced upregulation of DOPEY1 drives p53 degradation via the USP7/TRIP12 axis, contributing to gastric tumorigenesis, and highlight DOPEY1 as a potential therapeutic target for H. pylori-associated GC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets supporting the conclusions of this article are included within the article and additional files.
References
Amieva M, Peek RM Jr. Pathobiology of Helicobacter pylori-Induced Gastric Cancer. Gastroenterology. 2016;150:64–78.
Hu Y, Liu JP, Li XY, Cai Y, He C, Li NS, et al. Downregulation of tumor suppressor RACK1 by Helicobacter pylori infection promotes gastric carcinogenesis through the integrin beta-1/NF-kappaB signaling pathway. Cancer Lett. 2019;450:144–54.
Hu Y, He C, Liu JP, Li NS, Peng C, Yang-Ou YB, et al. Analysis of key genes and signaling pathways involved in Helicobacter pylori-associated gastric cancer based on The Cancer Genome Atlas database and RNA sequencing data. Helicobacter. 2018;23:e12530.
Hardbower DM, de Sablet T, Chaturvedi R, Wilson KT. Chronic inflammation and oxidative stress: the smoking gun for Helicobacter pylori-induced gastric cancer? Gut Microbes. 2013;4:475–81.
Muer A, Overkamp T, Gillissen B, Richter A, Pretzsch T, Milojkovic A, et al. p14(ARF)-induced apoptosis in p53 protein-deficient cells is mediated by BH3-only protein-independent derepression of Bak protein through down-regulation of Mcl-1 and Bcl-xL proteins. J Biol Chem. 2012;287:17343–52.
Collado M, Serrano M. The TRIP from ULF to ARF. Cancer Cell. 2010;17:317–8.
Chen D, Shan J, Zhu WG, Qin J, Gu W. Transcription-independent ARF regulation in oncogenic stress-mediated p53 responses. Nature. 2010;464:624–7.
Imai S, Ooki T, Murata-Kamiya N, Komura D, Tahmina K, Wu W, et al. Helicobacter pylori CagA elicits BRCAness to induce genome instability that may underlie bacterial gastric carcinogenesis. Cell Host Microbe. 2021;29:941–58.e910.
Wei J, Noto JM, Zaika E, Romero-Gallo J, Piazuelo MB, Schneider B, et al. Bacterial CagA protein induces degradation of p53 protein in a p14ARF-dependent manner. Gut. 2015;64:1040–8.
Horvat A, Noto JM, Ramatchandirin B, Zaika E, Palrasu M, Wei J, et al. Helicobacter pylori pathogen regulates p14ARF tumor suppressor and autophagy in gastric epithelial cells. Oncogene. 2018;37:5054–65.
Tanaka M, Izawa T, Yamate J, Franklin RJ, Kuramoto T, Serikawa T, et al. The VF rat with abnormal myelinogenesis has a mutation in Dopey1. Glia. 2014;62:1530–42.
Mahajan D, Tie HC, Chen B, Lu L. Dopey1-Mon2 complex binds to dual-lipids and recruits kinesin-1 for membrane trafficking. Nat Commun. 2019;10:3218.
Lend AK, Kazantseva A, Kivil A, Valvere V, Palm K. Diagnostic significance of alternative splice variants of REST and DOPEY1 in the peripheral blood of patients with breast cancer. Tumour Biol. 2015;36:2473–80.
Li N, Feng Y, Hu Y, He C, Xie C, Ouyang Y, et al. Helicobacter pylori CagA promotes epithelial mesenchymal transition in gastric carcinogenesis via triggering oncogenic YAP pathway. J Exp Clin Cancer Res. 2018;37:280.
Cai JB, Shi GM, Dong ZR, Ke AW, Ma HH, Gao Q, et al. Ubiquitin-specific protease 7 accelerates p14(ARF) degradation by deubiquitinating thyroid hormone receptor-interacting protein 12 and promotes hepatocellular carcinoma progression. Hepatology. 2015;61:1603–14.
Rogers AB, Taylor NS, Whary MT, Stefanich ED, Wang TC, Fox JG. Helicobacter pylori but not high salt induces gastric intraepithelial neoplasia in B6129 mice. Cancer Res. 2005;65:10709–15.
Li N, Xu X, Zhan Y, Fei X, Ouyang Y, Zheng P, et al. YAP and beta-catenin cooperate to drive H. pylori-induced gastric tumorigenesis. Gut Microbes. 2023;15:2192501.
Cao L, Zhu S, Lu H, Soutto M, Bhat N, Chen Z, et al. Helicobacter pylori-induced RASAL2 Through Activation of Nuclear Factor-kappaB Promotes Gastric Tumorigenesis via beta-catenin Signaling Axis. Gastroenterology. 2022;162:1716–31.e1717.
Zhou Y, Zhang W, He C, Shu C, Xu X, Wang H, et al. Metal-Organic Framework Based Mucoadhesive Nanodrugs for Multifunction Helicobacter Pylori Targeted Eradication, Inflammation Regulation and Gut Flora Protection. Small. 2024;20:e2308286.
Xie C, Li N, Wang H, He C, Hu Y, Peng C, et al. Inhibition of autophagy aggravates DNA damage response and gastric tumorigenesis via Rad51 ubiquitination in response to H. pylori infection. Gut Microbes. 2020;11:1567–89.
Infection with Helicobacter pylori. IARC Monogr Eval Carcinog Risks Hum. 1994;61:177–240.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
Plummer M, Franceschi S, Vignat J, Forman D, de Martel C. Global burden of gastric cancer attributable to Helicobacter pylori. Int J Cancer. 2015;136:487–90.
Wang H, Zhao M, Shi F, Zheng S, Xiong L, Zheng L. A review of signal pathway induced by virulent protein CagA of Helicobacter pylori. Front Cell Infect Microbiol. 2023;13:1062803.
Meng L, Shi H, Wang Z, Fan M, Pang S, Lin R. The Gamma-glutamyltransferase gene of Helicobacter pylori can promote gastric carcinogenesis by activating Wnt signal pathway through up-regulating TET1. Life Sci. 2021;267:118921.
Jiang X, Wang W, Wang Z, Wang Z, Shi H, Meng L, et al. Gamma-glutamyl transferase secreted by Helicobacter pylori promotes the development of gastric cancer by affecting the energy metabolism and histone methylation status of gastric epithelial cells. Cell Commun Signal. 2024;22:402.
Yong X, Tang B, Xiao YF, Xie R, Qin Y, Luo G, et al. Helicobacter pylori upregulates Nanog and Oct4 via Wnt/beta-catenin signaling pathway to promote cancer stem cell-like properties in human gastric cancer. Cancer Lett. 2016;374:292–303.
Liu B, Bukhari I, Li F, Ren F, Xia X, Hu B, et al. Enhanced LRP8 expression induced by Helicobacter pylori drives gastric cancer progression by facilitating beta-Catenin nuclear translocation. J Adv Res. 2024;S2090-1232:00131-0.
Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology. 2019;224:242–53.
Wrigley JD, Eckersley K, Hardern IM, Millard L, Walters M, Peters SW, et al. Enzymatic characterisation of USP7 deubiquitinating activity and inhibition. Cell Biochem Biophys. 2011;60:99–111.
Saha G, Roy S, Basu M, Ghosh MK. USP7 - a crucial regulator of cancer hallmarks. Biochim Biophys Acta Rev Cancer. 2023;1878:188903.
Dai X, Lu L, Deng S, Meng J, Wan C, Huang J, et al. USP7 targeting modulates anti-tumor immune response by reprogramming Tumor-associated Macrophages in Lung Cancer. Theranostics. 2020;10:9332–47.
Yi J, Li H, Chu B, Kon N, Hu X, Hu J, et al. Inhibition of USP7 induces p53-independent tumor growth suppression in triple-negative breast cancers by destabilizing FOXM1. Cell Death Differ. 2023;30:1799–810.
Zhang K, Sun T, Li W, Guo Y, Li A, Hsieh M, et al. Inhibition of USP7 upregulates USP22 and activates its downstream cancer-related signaling pathways in human cancer cells. Cell Commun Signal. 2023;21:319.
Cummins JM, Rago C, Kohli M, Kinzler KW, Lengauer C, Vogelstein B. Tumour suppression: disruption of HAUSP gene stabilizes p53. Nature. 2004;428:1.
Al-Eidan A, Wang Y, Skipp P, Ewing RM. The USP7 protein interaction network and its roles in tumorigenesis. Genes Dis. 2022;9:41–50.
Guan X, Wang Y, Yu W, Wei Y, Lu Y, Dai E, et al. Blocking Ubiquitin-Specific Protease 7 Induces Ferroptosis in Gastric Cancer via Targeting Stearoyl-CoA Desaturase. Adv Sci. 2024;11:e2307899.
Acknowledgements
We would like to thank Xi-Dong Wu (Department of Drug Safety Evaluation, Jiangxi Testing Center of Medical Instruments, Nanchang, China) for assistance with animal experiments.
Funding
This research was supported by National Natural Science Foundation of China, Grant/Award Number: Nos. 82000531, 82170580, and 82360118; Academic and Technical Leader Training Program of Major Disciplines in Jiangxi Province, China, Grant/Award Number: 20212BCJL23065; The Natural Science Foundation of Jiangxi Province, Grant/Award Number: 20242BAB25436; Jiangxi Provincial Health Technology Project, Grant/Award Number: 202410174.
Author information
Authors and Affiliations
Contributions
AZ performed this study and wrote the manuscript; AZ, YH, YZ, and NL conceived and designed the experiments; AZ, NL, YZ, ZH, LL, YO, YD and XW conducted in vivo and in vitro experiments; HZ, HW, XX, XF, and CH collected the samples and analyzed the data; YH, NL, YZ, and JL revised the manuscript; YH and YZ obtained funding of the study; All authors approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the ethics committee of The First Affiliated Hospital of Nanchang University ((2023) CDYFYYLK (01-009)), and written informed consent was obtained from all patients. The animal study was reviewed and approved by the ethics committees of Nanchang University (NCULAE-20221228028). All methods were performed in accordance with the relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhou, YA., Li, NS., Zhu, YC. et al. Helicobacter pylori activates DOPEY1 to promote p53 degradation through the USP7/TRIP12 axis in gastric tumorigenesis. Oncogene 44, 1245–1258 (2025). https://doi.org/10.1038/s41388-025-03303-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41388-025-03303-5