Abstract
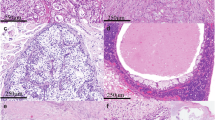
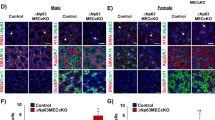
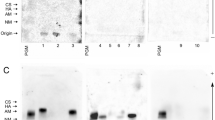
Mucoepidermoid carcinoma (MEC) is the most frequently occurring salivary gland malignancy. Here, we investigated transcriptomic profiles of human fetal and adult salivary glands and MEC tumors to assess programs involved in MEC progression. Molecular and genetic analyses revealed that MEC tumors and fetal salivary glands share proliferative and developmental gene expression profiles that implicate an FGFR-p53 signaling axis in salivary MEC progression. Based on these findings, we developed a genetically engineered mouse model of advanced MEC via targeted expression of the CRTC1-MAML2 oncogene in salivary ductal cells. Specifically, CRTC1-MAML2 expression combined with p53 dysregulation in salivary ducts rewires FGF signaling to drive formation of tumors with histological and molecular features of high-grade MEC. The combined bioinformatics and mouse modeling of this study demonstrate that salivary MEC progression is underpinned by reactivation of developmental signaling programs and suggests a role for FGFR targeted therapies in the treatment of high-grade MEC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are available in the NCBI Gene Expression Omnibus repository. The normalized gene expression data matrices and clinical annotation for this study are available at the Gene Expression Omnibus under GSE143702 and GSE282430.
Code availability
All computer code employed are commercially available. No custom computer code was generated or used for the analyses performed in this study.
Change history
16 June 2025
The original online version of this article was revised: In this article the author’s name Luane J. B. Landau was incorrectly written as Luane J. B. Landuau.
20 June 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41388-025-03478-x
References
Lee MY. Embryonic programs in cancer and metastasis-insights from the mammary gland. Front Cell Dev Biol. 2022;10:938625.
Coghe F, Fanni D, Gerosa C, Ravarino A, Mureddu M, Cerrone G, et al. The role of fetal programming in human carcinogenesis - may the Barker hypothesis explain interindividual variability in susceptibility to cancer insurgence and progression?. Eur Rev Med Pharm Sci. 2022;26:3585–92.
Balachandran S, Narendran A. The developmental origins of cancer: a review of the genes expressed in embryonic cells with implications for tumorigenesis. Genes. 2023;14:604.
Meacham CE, Morrison SJ. Tumour heterogeneity and cancer cell plasticity. Nature. 2013;501:328–37.
Junttila MR, de Sauvage FJ. Influence of tumour micro-environment heterogeneity on therapeutic response. Nature. 2013;501:346–54.
Stanta G, Bonin S. Overview on clinical relevance of intra-tumor heterogeneity. Front Med (Lausanne). 2018;5:85.
Dentro SC, Leshchiner I, Haase K, Tarabichi M, Wintersinger J, Deshwar AG, et al. Characterizing genetic intra-tumor heterogeneity across 2,658 human cancer genomes. Cell. 2021;184:2239–54.
Marusyk A, Janiszewska M, Polyak K. Intratumor heterogeneity: the Rosetta Stone of therapy resistance. Cancer Cell. 2020;37:471–84.
Gavish A, Tyler M, Greenwald AC, Hoefflin R, Simkin D, Tschernichovsky R, et al. Hallmarks of transcriptional intratumour heterogeneity across a thousand tumours. Nature. 2023;618:598–606.
Bleckmann SC, Blendy JA, Rudolph D, Monaghan AP, Schmid W, Schütz G. Activating transcription factor 1 and CREB are important for cell survival during early mouse development. Mol Cell Biol. 2002;22:1919–25.
Struthers RS, Vale WW, Arias C, Sawchenko PE, Montminy MR. Somatotroph hypoplasia and dwarfism in transgenic mice expressing a non-phosphorylatable CREB mutant. Nature. 1991;350:622–4.
Rosenberg D, Groussin L, Jullian E, Perlemoine K, Bertagna X, Bertherat J. Role of the PKA-regulated transcription factor CREB in development and tumorigenesis of endocrine tissues. Ann N Y Acad Sci. 2002;968:65–74.
Sundaram N, Tao Q, Wylie C, Heasman J. The role of maternal CREB in early embryogenesis of Xenopus laevis. Dev Biol. 2003;261:337–52.
Sakamoto KM, Frank DA. CREB in the pathophysiology of cancer: implications for targeting transcription factors for cancer therapy. Clin Cancer Res: J Am Assoc Cancer Res. 2009;15:2583–7.
Conkright M, Montminy M. CREB: the unindicted cancer co-conspirator. Trends Cell Biol. 2005;15:457–9.
Steven A, Heiduk M, Recktenwald CV, Hiebl B, Wickenhauser C, Massa C, et al. Colorectal Carcinogenesis: Connecting K-RAS-Induced Transformation and CREB Activity In Vitro and In Vivo. Mol Cancer Res. 2015;13:1248–62.
Li B, Zheng L, Ye J, Zhang C, Zhou J, Huang Q, et al. CREB1 contributes colorectal cancer cell plasticity by regulating lncRNA CCAT1 and NF-kappaB pathways. Sci China Life Sci. 2022;65:1481–97.
Luna MA. Salivary mucoepidermoid carcinoma: revisited. Adv Anat Pathol. 2006;13:293–307.
O’Neill ID. t(11;19) translocation and CRTC1-MAML2 fusion oncogene in mucoepidermoid carcinoma. Oral Oncol. 2009;45:2–9.
Chen Z, Ni W, Li JL, Lin S, Zhou X, Sun Y. he CRTC1-MAML2 fusion is the major oncogenic driver in mucoepidermoid carcinoma. JCI Insight. 2021;6:e139497.
Chen J, Li JL, Chen Z, Griffin JD, Wu L. Gene expression profiling analysis of CRTC1-MAML2 fusion oncogene-induced transcriptional program in human mucoepidermoid carcinoma cells. BMC Cancer. 2015;15:803.
Wu L, Liu J, Gao P, Nakamura M, Cao Y, Shen H, et al. Transforming activity of MECT1-MAML2 fusion oncoprotein is mediated by constitutive CREB activation. EMBO J. 2005;24:2391–402.
Komiya T, Park Y, Modi S, Coxon AB, Oh H, Kaye FJ. Sustained expression of Mect1-Maml2 is essential for tumor cell growth in salivary gland cancers carrying the t(11;19) translocation. Oncogene. 2006;25:6128–32.
Amelio AL, Fallahi M, Schaub FX, Zhang M, Lawani MB, Alperstein AS, et al. CRTC1/MAML2 gain-of-function interactions with MYC create a gene signature predictive of cancers with CREB-MYC involvement. Proc Natl Acad Sci USA. 2014;111:E3260–E8.
Musicant AM, Parag-Sharma K, Gong W, Sengupta M, Chatterjee A, Henry EC, et al. CRTC1/MAML2 directs a PGC-1alpha-IGF-1 circuit that confers vulnerability to PPARgamma inhibition. Cell Rep. 2021;34:108768.
Batsakis JG. Salivary gland neoplasia: an outcome of modified morphogenesis and cytodifferentiation. Oral Surg Oral Med Oral Pathol. 1980;49:229–32.
Brandwein MS, Ivanov K, Wallace DI, Hille JJ, Wang B, Fahmy A, et al. Mucoepidermoid carcinoma: a clinicopathologic study of 80 patients with special reference to histological grading. Am J Surg Pathol. 2001;25:835–45.
Goode RK, Auclair PL, Ellis GL. Mucoepidermoid carcinoma of the major salivary glands: clinical and histopathologic analysis of 234 cases with evaluation of grading criteria. Cancer. 1998;82:1217–24.
Katabi N, Ghossein R, Ali S, Dogan S, Klimstra D, Ganly I. Prognostic features in mucoepidermoid carcinoma of major salivary glands with emphasis on tumour histologic grading. Histopathology. 2014;65:793–804.
Hicks MJ, el-Naggar AK, Byers RM, Flaitz CM, Luna MA, Batsakis JG. Prognostic factors in mucoepidermoid carcinomas of major salivary glands: a clinicopathologic and flow cytometric study. Eur J Cancer B Oral Oncol. 1994;30B:329–34.
Spiro RH, Huvos AG, Berk R, Strong EW. Mucoepidermoid carcinoma of salivary-gland origin - clinicopathologic study of 367 cases. Am J Surg. 1978;136:461–8.
Fonseca I, Clode AL, Soares J. Mucoepidermoid carcinoma of major and minor salivary glands: a survey of 43 cases with study of prognostic indicators. Int J Surg Pathol. 1993;1:3–12.
Saitou M, Gaylord EA, Xu E, May AJ, Neznanova L, Nathan S, et al. Functional specialization of human salivary glands and origins of proteins intrinsic to human saliva. Cell Rep. 2020;33:108402.
Chen S, Zhou Y, Chen Y, Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics. 2018;34:i884–i90.
Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21.
Patro R, Duggal G, Love MI, Irizarry RA, Kingsford C. Salmon provides fast and bias-aware quantification of transcript expression. Nat Methods. 2017;14:417–9.
Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550.
Huber W, Carey VJ, Gentleman R, Anders S, Carlson M, Carvalho BS, et al. Orchestrating high-throughput genomic analysis with Bioconductor. Nat Methods. 2015;12:115–21.
Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–50.
Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–25.
Grossman RL, Heath AP, Ferretti V, Varmus HE, Lowy DR, Kibbe WA, et al. Toward a shared vision for cancer genomic data. N Engl J Med. 2016;375:1109–12.
Shen S, Park JW, Lu ZX, Lin L, Henry MD, Wu YN, et al. rMATS: robust and flexible detection of differential alternative splicing from replicate RNA-Seq data. Proc Natl Acad Sci. 2014;111:E5593–E601.
Ramirez F, Ryan DP, Gruning B, Bhardwaj V, Kilpert F, Richter AS, et al. deepTools2: a next generation web server for deep-sequencing data analysis. Nucleic Acids Res. 2016;44:W160–W5.
Roulis M, Nikolaou C, Kotsaki E, Kaffe E, Karagianni N, Koliaraki V, et al. Intestinal myofibroblast-specific Tpl2-Cox-2-PGE2 pathway links innate sensing to epithelial homeostasis. Proc Natl Acad Sci USA. 2014;111:E4658–E67.
Blum R, Gupta R, Burger PE, Ontiveros CS, Salm SN, Xiong X, et al. Molecular signatures of prostate stem cells reveal novel signaling pathways and provide insights into prostate cancer. PLoS One. 2009;4:e5722.
Damrauer JS, Roell KR, Smith MA, Sun X, Kirk EL, Hoadley KA, et al. Identification of a Novel Inflamed Tumor Microenvironment Signature as a Predictive Biomarker of Bacillus Calmette-Guerin Immunotherapy in Non-Muscle-Invasive Bladder Cancer. Clin Cancer Res. 2021;27:4599–609.
Maruyama EO, Aure MH, Xie X, Myal Y, Gan L, Ovitt CE. Cell-specific Cre strains for genetic manipulation in salivary glands. PLoS One. 2016;11:e0146711.
Shi G, Zhu L, Sun Y, Bettencourt R, Damsz B, Hruban RH, et al. Loss of the acinar-restricted transcription factor Mist1 accelerates Kras-induced pancreatic intraepithelial neoplasia. Gastroenterology. 2009;136:1368–78.
Schaub FX, Reza MS, Flaveny CA, Li W, Musicant AM, Hoxha S, et al. Fluorophore-NanoLuc BRET reporters enable sensitive in vivo optical imaging and flow cytometry for monitoring tumorigenesis. Cancer Res. 2015;75:5023–33.
Carper MB, Troutman S, Wagner BL, Byrd KM, Selitsky SR, Parag-Sharma K, et al. An immunocompetent mouse model of HPV16(+) head and neck squamous cell carcinoma. Cell Rep. 2019;29:1660–74.
Detre S, Saclani Jotti G, Dowsett M. A “quickscore” method for immunohistochemical semiquantitation: validation for oestrogen receptor in breast carcinomas. J Clin Pathol. 1995;48:876–8.
Tonon G, Modi S, Wu L, Kubo A, Coxon A, Komiya T, et al. t(11;19)(q21;p13) translocation in mucoepidermoid carcinoma creates a novel fusion product that disrupts a Notch signaling pathway. Nat Genet. 2003;33:208–13.
Young L, Sung J, Stacey G, Masters JR. Detection of mycoplasma in cell cultures. Nat Protoc. 2010;5:929–34.
Spike BT, Engle DD, Lin JC, Cheung SK, La J, Wahl GM. A mammary stem cell population identified and characterized in late embryogenesis reveals similarities to human breast cancer. Cell Stem Cell. 2012;10:183–97.
Stanger BZ, Wahl GM. Cancer as a disease of development gone awry. Annu Rev Pathol. 2024;19:397–421.
Sole L, Lobo-Jarne T, Alvarez-Villanueva D, Alonso-Maranon J, Guillen Y, Guix M, et al. p53 wild-type colorectal cancer cells that express a fetal gene signature are associated with metastasis and poor prognosis. Nat Commun. 2022;13:2866.
Janky R, Verfaillie A, Imrichova H, Van de Sande B, Standaert L, Christiaens V, et al. iRegulon: from a gene list to a gene regulatory network using large motif and track collections. PLoS Comput Biol. 2014;10:e1003731.
Pandit B, Halasi M, Gartel AL. p53 negatively regulates expression of FoxM1. Cell Cycle. 2009;8:3425–7.
Barsotti AM, Prives C. Pro-proliferative FoxM1 is a target of p53-mediated repression. Oncogene. 2009;28:4295–305.
Hingorani SR, Wang L, Multani AS, Combs C, Deramaudt TB, Hruban RH, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–83.
Wang K, McDermott JD, Schrock AB, Elvin JA, Gay L, Karam SD, et al. Comprehensive genomic profiling of salivary mucoepidermoid carcinomas reveals frequent BAP1, PIK3CA, and other actionable genomic alterations. Ann Oncol. 2017;28:748–53.
Wang X, Bai J, Yan J, Li B. The clinical outcome, pathologic spectrum, and genomic landscape for 454 cases of salivary mucoepidermoid carcinoma. NPJ Precis Oncol. 2024;8:238.
Gomes CC, Diniz MG, Orsine LA, Duarte AP, Fonseca-Silva T, Conn BI, et al. Assessment of TP53 mutations in benign and malignant salivary gland neoplasms. PLoS One. 2012;7:e41261.
Rodriguez-Ramirez C, Zhang Z, Warner KA, Herzog AE, Mantesso A, Zhang Z, et al. p53 inhibits Bmi-1-driven self-renewal and defines salivary gland cancer stemness. Clin Cancer Res. 2022;28:4757–70.
Andrews A, Warner K, Rodriguez-Ramirez C, Pearson AT, Nor F, Zhang Z, et al. Ablation of cancer stem cells by therapeutic inhibition of the MDM2-p53 interaction in mucoepidermoid carcinoma. Clin Cancer Res. 2019;25:1588–600.
Jaskoll T, Htet K, Abichaker G, Kaye FJ, Melnick M. CRTC1 expression during normal and abnormal salivary gland development supports a precursor cell origin for mucoepidermoid cancer. Gene Expr Patterns. 2011;11:57–63.
Adams A, Warner K, Pearson AT, Zhang Z, Kim HS, Mochizuki D, et al. ALDH/CD44 identifies uniquely tumorigenic cancer stem cells in salivary gland mucoepidermoid carcinomas. Oncotarget. 2015;6:26633–50.
Ninche N, Kwak M, Ghazizadeh S Diverse epithelial cell populations contribute to the regeneration of secretory units in injured salivary glands. Development. 2020;147.
Batsakis JG, Regezi JA, Luna MA, el-Naggar A. Histogenesis of salivary gland neoplasms: a postulate with prognostic implications. J Laryngol Otol. 1989;103:939–44.
May AJ, Cruz-Pacheco N, Emmerson E, Gaylord EA, Seidel K, Nathan S, et al. Diverse progenitor cells preserve salivary gland ductal architecture after radiation-induced damage. Development. 2018;145.
Aure MH, Konieczny SF, Ovitt CE. Salivary gland homeostasis is maintained through acinar cell self-duplication. Dev Cell. 2015;33:231–7.
Vasioukhin V, Degenstein L, Wise B, Fuchs E. The magical touch: genome targeting in epidermal stem cells induced by tamoxifen application to mouse skin. Proc Natl Acad Sci USA. 1999;96:8551–6.
Nitta M, Kume T, Nogawa H. FGF alters epithelial competence for EGF at the initiation of branching morphogenesis of mouse submandibular gland. Dev Dynam. 2009;238:315–23.
Aure MH, Symonds JM, Villapudua CU, Dodge JT, Werner S, Knosp WM, et al. FGFR2 is essential for salivary gland duct homeostasis and MAPK-dependent seromucous acinar cell differentiation. Nat Commun. 2023;14:6485.
Mattingly A, Finley JK, Knox SM. Salivary gland development and disease. Wiley Interdiscip Rev Dev Biol. 2015;4:573–90.
Xie Y, Su N, Yang J, Tan Q, Huang S, Jin M, et al. FGF/FGFR signaling in health and disease. Signal Transduct Target Ther. 2020;5:181.
Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat Rev Drug Discov. 2009;8:235–53.
Wang S, Sekiguchi R, Daley WP, Yamada KM. Patterned cell and matrix dynamics in branching morphogenesis. J Cell Biol. 2017;216:559–70.
Hsu JC, Yamada KM. Salivary gland branching morphogenesis-recent progress and future opportunities. Int J Oral Sci. 2010;2:117–26.
Musselmann K, Green JA, Sone K, Hsu JC, Bothwell IR, Johnson SA, et al. Salivary gland gene expression atlas identifies a new regulator of branching morphogenesis. J Dent Res. 2011;90:1078–84.
Yeh BK, Igarashi M, Eliseenkova AV, Plotnikov AN, Sher I, Ron D, et al. Structural basis by which alternative splicing confers specificity in fibroblast growth factor receptors. Proc Natl Acad Sci USA. 2003;100:2266–71.
Finburgh EN, Mauduit O, Noguchi T, Bu JJ, Abbas AA, Hakim DF, et al. Role of FGF10/FGFR2b Signaling in Homeostasis and Regeneration of Adult Lacrimal Gland and Corneal Epithelium Proliferation. Invest Ophthalmol Vis Sci. 2023;64:21.
Epstein RJ, Tian LJ, Gu YF. 2b or not 2b: how opposing FGF receptor splice variants are blocking progress in precision oncology. J Oncol. 2021;2021:9955456.
Holzmann K, Grunt T, Heinzle C, Sampl S, Steinhoff H, Reichmann N, et al. Alternative Splicing of Fibroblast Growth Factor Receptor IgIII Loops in Cancer. J Nucleic Acids. 2012;2012:950508.
Amelio AL, Caputi M, Conkright MD. Bipartite functions of the CREB co-activators selectively direct alternative splicing or transcriptional activation. EMBO J. 2009;28:2733–47.
Tasoulas J, Srivastava S, Xu X, Tarasova V, Maniakas A, Karreth FA, et al. Genetically engineered mouse models of head and neck cancers. Oncogene. 2023;42:2593–609.
Lombaert IM, Abrams SR, Li L, Eswarakumar VP, Sethi AJ, Witt RL, et al. Combined KIT and FGFR2b signaling regulates epithelial progenitor expansion during organogenesis. Stem Cell Rep. 2013;1:604–19.
Emmerson E, Knox SM. Salivary gland stem cells: a review of development, regeneration and cancer. Genesis. 2018;56:e23211.
Chatzeli L, Bordeu I, Han S, Bisetto S, Waheed Z, Koo BK, et al. A cellular hierarchy of Notch and Kras signaling controls cell fate specification in the developing mouse salivary gland. Dev Cell. 2023;58:94–109.
Mantovani F, Collavin L, Del Sal G. Mutant p53 as a guardian of the cancer cell. Cell Death Differ. 2019;26:199–212.
Bieging KT, Mello SS, Attardi LD. Unravelling mechanisms of p53-mediated tumour suppression. Nat Rev Cancer. 2014;14:359–70.
Rodriguez-Ramirez C, Nor JE. p53 and cell fate: sensitizing head and neck cancer stem cells to chemotherapy. Crit Rev Oncog. 2018;23:173–87.
Kiyoshima T, Shima K, Kobayashi I, Matsuo K, Okamura K, Komatsu S, et al. Expression of p53 tumor suppressor gene in adenoid cystic and mucoepidermoid carcinomas of the salivary glands. Oral Oncol. 2001;37:315–22.
Katoh M, Loriot Y, Brandi G, Tavolari S, Wainberg ZA, Katoh M. FGFR-targeted therapeutics: clinical activity, mechanisms of resistance and new directions. Nat Rev Clin Oncol. 2024;21:312–29.
Shoji K, Teishima J, Hayashi T, Ohara S, McKeehan WL, Matsubara A. Restoration of fibroblast growth factor receptor 2IIIb enhances the chemosensitivity of human prostate cancer cells. Oncol Rep. 2014;32:65–70.
Matsubara A, Teishima J, Mirkhat S, Yasumoto H, Mochizuki H, Seki M, et al. Restoration of FGF receptor type 2 enhances radiosensitivity of hormone-refractory human prostate carcinoma PC-3 cells. Anticancer Res. 2008;28:2141–6.
Varghese JJ, Schmale IL, Wang Y, Hansen ME, Newlands SD, Ovitt CE, et al. Retroductal nanoparticle injection to the murine submandibular gland. J Vis Exp. 2018;e7521. https://doi.org/10.3791/57521.
Troester MA, Herschkowitz JI, Oh DS, He X, Hoadley KA, Barbier CS, et al. Gene expression patterns associated with p53 status in breast cancer. BMC Cancer. 2006;6:276.
Acknowledgements
We are grateful for the support of previous and current members of the Amelio lab, with special thanks to Dr. Harish Bharambe for his technical expertise and assistance. We would also like to thank Gabriela De La Cruz and Bentley Midkiff in the Pathology Services Core and David Corcoran in the Lineberger Bioinformatics Core of the University of North Carolina at Chapel Hill for expert technical assistance with histological staining and fluorescent imaging. The Pathology Services Core is supported in part by an NCI Center Core Support Grant (P30-CA016086). In addition, the authors would like to acknowledge Dr. Jimena Guidice and Dr. Jessica Cote for their technical assistance. This work was also supported in part by Dr. Joseph Johnson and Brooke Smedley of the Analytic Microscopy Core, Jodi Balasi of the Tissue Core, and Dr. Mikalai Budzevich and Epi Ruiz of the Small Animal Imaging Lab at the Moffitt Cancer Center and Research Institute, a comprehensive cancer center designated by the National Cancer Institute and funded in part by Moffitt’s Cancer Center Support Grant (P30-CA076292). This work was supported in part by NIH/NIGMS T32-GM007092 and NIH/NIDCR F31-DE027282 training grants (to AMM), Head and Neck Cancer Fund (to TGH and DNH), NIH/NCATS-supported UL1-TR002489 UNC Translational Team Science Award (TTSA#026P1; to TGH and ALA), Moffitt Cancer Center funds (to ALA), and NIH/NIDCR R01-DE030123 (to ALA).
Author information
Authors and Affiliations
Contributions
Conception and design: AMM, JMRB, and ALA. Development of methodology: AMM, JMRB, JSD, JHM, LJBL, Y-HT, and ALA. Acquisition of data (provided reagents, provided facilities, etc.): AMM, JMRB, JP, RB, and RS. Interpretation of data (e.g., statistical analysis, biostatistics, computational analysis): AMM, JMRB, JSD, JHM, LJBL, Y-HT, JM, OG, SMK, and ALA. Writing of the manuscript: AMM, JMRB, and ALA. Review and revision of the manuscript: AMM, JMRB, JSD, JHM, LJBL, Y-HT, JM, JP, RB, RS, RJP, JCH-P, DNH, TGH, OG, SMK, and ALA. Administrative, technical, or material support (i.e., reporting or organizing data, constructing databases): JM, LJBL, Y-HT, JHM, RB, RS, OG, SMK, and ALA. Study supervision: OG, SMK, and ALA. Acquisition of funding: TGH and ALA.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
Research involving human tissues was reviewed and approved by the Institutional Review Boards at The University of North Carolina at Chapel Hill (IRB protocols 15-1604 and 17-2947) and University of California—San Francisco (IRB protocol 10-00768) and informed consent was obtained from all participants. Research involving murine tissues was reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of The University of North Carolina Chapel Hill (IACUC protocols 17-202 and 20-142), Moffitt Cancer Center and the University of South Florida (IACUC protocols 11291 M and 11379 R). All methods were performed in accordance with relevant guidelines and regulations.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Musicant, A.M., Billington, J.M.R., Damrauer, J.S. et al. An FGFR-p53 developmental signaling axis drives salivary cancer progression. Oncogene (2025). https://doi.org/10.1038/s41388-025-03444-7
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-025-03444-7