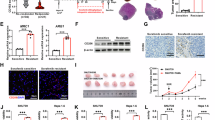
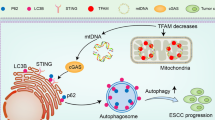
Abstract
The infiltration of TAMs mediates an immunosuppressive tumor microenvironment, which plays a crucial role in the malignant progression of HCC. TFAM is a key molecule that regulates mtDNA replication and transcription, and its expression frequently downregulated in various tumors, including HCC. Our previous study indicated that the downregulation of TFAM triggers mtDNA stress, thereby inducing autophagy and promoting ESCC survival through the STING pathway. However, it remains unclear whether cytosolic mtDNA stress, mediated by TFAM downregulation, is implicated in microenvironment regulation, particularly in the infiltration of TAMs in HCC. In this study, we found that TFAM expression was significantly decreased and correlated with CD163 expression in HCC tissues. The downregulated expression of TFAM in HCC cells contributed to TAM infiltration. Mechanistically, the downregulation of TFAM induced cytosolic mtDNA stress, which activated the NLRP3 inflammasome and promoted the expression of IL-18 and IL-1β in HCC cells, thereby inducing macrophage recruitment and M2 polarization. Depleting cytosolic mtDNA using DNase I or blocking NLRP3 inflammasome activation with an NLRP3 antagonist in HCC cells with TFAM downregulation significantly suppresses the infiltration of M2-TAMs. Moreover, blocking the mtDNA-NLRP3 pathway significantly inhibited TAM infiltration and orthotopic mouse HCC model progression. Taken together, our results reveal a novel mechanism by which cytosolic mtDNA stress mediates TAM infiltration in HCC.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
269,00 € per year
only 5,38 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available on request from the corresponding author Dengke Bao ([email protected]).
Code availability
The data that support the findings of this study are available on request from the corresponding author Dengke Bao ([email protected]).
References
Xu T, Yu S, Zhang J, Wu S. Dysregulated tumor-associated macrophages in carcinogenesis, progression and targeted therapy of gynecological and breast cancers. J Hematol Oncol. 2021;14:181. https://doi.org/10.1186/s13045-021-01198-9.
Genard G, Lucas S, Michiels C. Reprogramming of Tumor-Associated Macrophages with Anticancer Therapies: Radiotherapy versus Chemo- and Immunotherapies. Front Immunol. 2017;8:828. https://doi.org/10.3389/fimmu.2017.00828.
Zhang Q, He Y, Luo N, Patel SJ, Han Y, Gao R, et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell. 2019;179:829–845.e820. https://doi.org/10.1016/j.cell.2019.10.003.
Chew V, Lai L, Pan L, Lim CJ, Li J, Ong R, et al. Delineation of an immunosuppressive gradient in hepatocellular carcinoma using high-dimensional proteomic and transcriptomic analyses. Proc Natl Acad Sci USA. 2017;114:E5900–E5909. https://doi.org/10.1073/pnas.1706559114.
Zhou J, Wang W, Li Q. Potential therapeutic targets in the tumor microenvironment of hepatocellular carcinoma: reversing the protumor effect of tumor-associated macrophages. J Exp Clin Cancer Res. 2021;40:73. https://doi.org/10.1186/s13046-021-01873-2.
Liu YT, Mao ZW, Ding Y, Wang WL. Macrophages as targets in hepatocellular carcinoma therapy. Mol Cancer Ther. 2024;23:780–90. https://doi.org/10.1158/1535-7163.MCT-23-0660.
Boulton DP, Caino MC. Mitochondrial fission and fusion in tumor progression to metastasis. Front Cell Dev Biol. 2022;10:849962. https://doi.org/10.3389/fcell.2022.849962.
Diaz-Vegas A, Sanchez-Aguilera P, Krycer JR, Morales PE, Monsalves-Alvarez M, Cifuentes M, et al. Is Mitochondrial Dysfunction a Common Root of Noncommunicable Chronic Diseases? Endocr Rev 2020; 41. https://doi.org/10.1210/endrev/bnaa005.
Vyas S, Zaganjor E, Haigis MC. Mitochondria and Cancer. Cell. 2016;166:555–66. https://doi.org/10.1016/j.cell.2016.07.002.
Weinberg SE, Sena LA, Chandel NS. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42:406–17. https://doi.org/10.1016/j.immuni.2015.02.002.
Huang Y, Zhou JH, Zhang H, Canfran-Duque A, Singh AK, Perry RJ, et al. Brown adipose TRX2 deficiency activates mtDNA-NLRP3 to impair thermogenesis and protect against diet-induced insulin resistance. J Clin Invest 2022; 132. https://doi.org/10.1172/JCI148852.
Jentho E, Weis S. DAMPs and innate immune training. Front Immunol. 2021;12:699563. https://doi.org/10.3389/fimmu.2021.699563.
Bao D, Zhao J, Zhou X, Yang Q, Chen Y, Zhu J, et al. Mitochondrial fission-induced mtDNA stress promotes tumor-associated macrophage infiltration and HCC progression. Oncogene. 2019;38:5007–20. https://doi.org/10.1038/s41388-019-0772-z.
Campbell CT, Kolesar JE, Kaufman BA. Mitochondrial transcription factor A regulates mitochondrial transcription initiation, DNA packaging, and genome copy number. Biochim Biophys Acta. 2012;1819:921–9. https://doi.org/10.1016/j.bbagrm.2012.03.002.
West AP, Khoury-Hanold W, Staron M, Tal MC, Pineda CM, Lang SM, et al. Mitochondrial DNA stress primes the antiviral innate immune response. Nature. 2015;520:553–7. https://doi.org/10.1038/nature14156.
West AP, Shadel GS. Mitochondrial DNA in innate immune responses and inflammatory pathology. Nat Rev Immunol. 2017;17:363–75. https://doi.org/10.1038/nri.2017.21.
Huang Q, Wu D, Zhao J, Yan Z, Chen L, Guo S, et al. TFAM loss induces nuclear actin assembly upon mDia2 malonylation to promote liver cancer metastasis. EMBO J. 2022;41:e110324. https://doi.org/10.15252/embj.2021110324.
Li Y, Yang Q, Chen H, Yang X, Han J, Yao X, et al. TFAM downregulation promotes autophagy and ESCC survival through mtDNA stress-mediated STING pathway. Oncogene. 2022;41:3735–46. https://doi.org/10.1038/s41388-022-02365-z.
Li Y, Chen H, Yang Q, Wan L, Zhao J, Wu Y, et al. Increased Drp1 promotes autophagy and ESCC progression by mtDNA stress mediated cGAS-STING pathway. J Exp Clin Cancer Res. 2022;41:76 https://doi.org/10.1186/s13046-022-02262-z.
Brinkkoetter PT, Bork T, Salou S, Liang W, Mizi A, Ozel C, et al. Anaerobic Glycolysis Maintains the Glomerular Filtration Barrier Independent of Mitochondrial Metabolism and Dynamics. Cell Rep. 2019;27:1551–1566.e1555. https://doi.org/10.1016/j.celrep.2019.04.012.
Zhao M, Wang Y, Li L, Liu S, Wang C, Yuan Y, et al. Mitochondrial ROS promote mitochondrial dysfunction and inflammation in ischemic acute kidney injury by disrupting TFAM-mediated mtDNA maintenance. Theranostics. 2021;11:1845–63. https://doi.org/10.7150/thno.50905.
Xu H, Chen J, Chen P, Li W, Shao J, Hong S, et al. Costunolide covalently targets NACHT ___domain of NLRP3 to inhibit inflammasome activation and alleviate NLRP3-driven inflammatory diseases. Acta Pharm Sin B. 2023;13:678–93. https://doi.org/10.1016/j.apsb.2022.09.014.
Blevins HM, Xu Y, Biby S, Zhang S. The NLRP3 Inflammasome pathway: a review of mechanisms and inhibitors for the treatment of inflammatory diseases. Front Aging Neurosci. 2022;14:879021. https://doi.org/10.3389/fnagi.2022.879021.
He Y, Hara H, Nunez G. Mechanism and regulation of NLRP3 inflammasome activation. Trends Biochem Sci. 2016;41:1012–21. https://doi.org/10.1016/j.tibs.2016.09.002.
Sanchez-Lopez E, Zhong Z, Stubelius A, Sweeney SR, Booshehri LM, Antonucci L, et al. Choline Uptake and Metabolism Modulate Macrophage IL-1beta and IL-18 Production. Cell Metab. 2019;29:1350–1362.e1357. https://doi.org/10.1016/j.cmet.2019.03.011.
Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589–604. https://doi.org/10.1038/s41575-019-0186-y.
Yang S, He X, Zhao J, Wang D, Guo S, Gao T, et al. Mitochondrial transcription factor A plays opposite roles in the initiation and progression of colitis-associated cancer. Cancer Commun. 2021;41:695–714. https://doi.org/10.1002/cac2.12184.
Si L, Fu J, Liu W, Hayashi T, Nie Y, Mizuno K, et al. Silibinin inhibits migration and invasion of breast cancer MDA-MB-231 cells through induction of mitochondrial fusion. Mol Cell Biochem. 2020;463:189–201. https://doi.org/10.1007/s11010-019-03640-6.
Chang TC, Lee HT, Pan SC, Cho SH, Cheng C, Ou LH, et al. Metabolic Reprogramming in response to alterations of mitochondrial DNA and mitochondrial dysfunction in gastric adenocarcinoma. Int J Mol Sci 2022; 23. https://doi.org/10.3390/ijms23031857.
Wu Q, Tsai HI, Zhu H, Wang D. The entanglement between mitochondrial DNA and tumor metastasis. Cancers 2022;14. https://doi.org/10.3390/cancers14081862.
Hsieh YT, Tu HF, Yang MH, Chen YF, Lan XY, Huang CL, et al. Mitochondrial genome and its regulator TFAM modulates head and neck tumourigenesis through intracellular metabolic reprogramming and activation of oncogenic effectors. Cell Death Dis. 2021;12:961. https://doi.org/10.1038/s41419-021-04255-w.
Gao X, Zhu B, Wu Y, Li C, Zhou X, Tang J, et al. TFAM-Dependent mitochondrial metabolism is required for alveolar macrophage maintenance and homeostasis. J Immunol. 2022;208:1456–66. https://doi.org/10.4049/jimmunol.2100741.
Li Z, Li X, Lu Y, Zhu X, Zheng W, Chen K, et al. Novel Photo-STING Agonists Delivered by Erythrocyte Efferocytosis-Mimicking Pattern to Repolarize Tumor-Associated Macrophages for Boosting Anticancer Immunotherapy. Adv Mater. 2024;36:e2410937. https://doi.org/10.1002/adma.202410937.
Liu J, Xiang J, Jin C, Ye L, Wang L, Gao Y, et al. Medicinal plant-derived mtDNA via nanovesicles induces the cGAS-STING pathway to remold tumor-associated macrophages for tumor regression. J Nanobiotechnol. 2023;21:78. https://doi.org/10.1186/s12951-023-01835-0.
Missiroli S, Perrone M, Boncompagni C, Borghi C, Campagnaro A, Marchetti F, et al. Targeting the NLRP3 inflammasome as a new therapeutic option for overcoming cancer. Cancers 2021;13. https://doi.org/10.3390/cancers13102297.
Ramos-Tovar E, Muriel P. NLRP3 inflammasome in hepatic diseases: A pharmacological target. Biochem Pharm. 2023;217:115861. https://doi.org/10.1016/j.bcp.2023.115861.
Xian H, Watari K, Sanchez-Lopez E, Offenberger J, Onyuru J, Sampath H, et al. Oxidized DNA fragments exit mitochondria via mPTP- and VDAC-dependent channels to activate NLRP3 inflammasome and interferon signaling. Immunity. 2022;55:1370–1385.e1378. https://doi.org/10.1016/j.immuni.2022.06.007.
Yu CH, Davidson S, Harapas CR, Hilton JB, Mlodzianoski MJ, Laohamonthonkul P, et al. TDP-43 Triggers Mitochondrial DNA Release via mPTP to Activate cGAS/STING in ALS. Cell. 2020;183:636–649.e618. https://doi.org/10.1016/j.cell.2020.09.020.
Jiang T, Liu E, Li Z, Yan C, Zhang X, Guan J, et al. SIRT1-Rab7 axis attenuates NLRP3 and STING activation through late endosomal-dependent mitophagy during sepsis-induced acute lung injury. Int J Surg. 2024;110:2649–68. https://doi.org/10.1097/JS9.0000000000001215.
Laube BL, Auci RM, Shields DE, Christiansen DH, Lucas MK, Fuchs HJ, et al. Effect of rhDNase on airflow obstruction and mucociliary clearance in cystic fibrosis. Am J Respir Crit Care Med. 1996;153:752–60. https://doi.org/10.1164/ajrccm.153.2.8564129.
Guichard MJ, Patil HP, Koussoroplis SJ, Wattiez R, Leal T, Vanbever R. Production and characterization of a PEGylated derivative of recombinant human deoxyribonuclease I for cystic fibrosis therapy. Int J Pharm. 2017;524:159–67. https://doi.org/10.1016/j.ijpharm.2017.03.057.
Huang W, Wen L, Tian H, Jiang J, Liu M, Ye Y, et al. Self-Propelled Proteomotors with Active Cell-Free mtDNA clearance for enhanced therapy of sepsis-associated acute lung injury. Adv Sci. 2023;10:e2301635. https://doi.org/10.1002/advs.202301635.
Chen J, Wang T, Li X, Gao L, Wang K, Cheng M, et al. DNA of neutrophil extracellular traps promote NF-kappaB-dependent autoimmunity via cGAS/TLR9 in chronic obstructive pulmonary disease. Signal Transduct Target Ther. 2024;9:163. https://doi.org/10.1038/s41392-024-01881-6.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant number 82373070 and 82002511). The Youth Promotion Project of Zhongzhou Laboratory for Integrative Biology (grant number 2024TS0101 and 2024TS0106). Postdoctoral Fellowship Program of CPSF (grant number GZC20230692), Excellent Youth Fund Project of Henan Natural Science Foundation (grant number 232300421043), Scientific and Technological Project of Henan Province in China (grant number SBGJ202302086 and 242102311046) and young backbone teachers of colleges and universities in Henan province (grant number 2021GGJS027), and Program for Central Plains Youth Top Talent for DKB, and Henan Postdoctoral Scientific Research Program (HN2025014).
Author information
Authors and Affiliations
Contributions
DKB and JZ performed the experiments and data analysis. JZ and XJY wrote this article. This article was revised by LXW, QY. HLL and MMC conceived and designed the study. ZXJ, LXQ, JXC., CHF, SWB, CYZ and RW helped with the data analysis. All authors read and approved the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, J., Cui, M., Yao, X. et al. Targeting TFAM downregulation mediated mtDNA-NLRP3 pathway suppresses TAM infiltration and HCC progression. Oncogene (2025). https://doi.org/10.1038/s41388-025-03467-0
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41388-025-03467-0