Abstract
Background
The Stockholm3 test improves Gleason Grade Group ≥2 (GG ≥ 2) prostate cancer (PC) detection, however it has not been evaluated in an American cohort where clinical practice patterns and ethnicity differ. We aimed to identify subgroups within a Stockholm population with PC risk profiles matching American ethnicity-specific subgroups and compare the detection of PC and describe Stockholm3 performance within these subgroups.
Methods
All men age 49–70 years presenting for prostate biopsies were evaluated at UIC from 2016 to 2019, as well as men in Stockholm from 2012 to 2014 in the STHLM3 study. Propensity scores (PS) were estimated for each person using logistic regression for age, PSA, prostate volume, family history of PC, 5-alpha reductase inhibitor use, and prior biopsy. 3:1 PS matching was performed for Stockholm to Chicago ethnicity-specific cohorts and odds ratios (OR) were computed to compare detection of GG ≥ 2 PC between groups.
Results
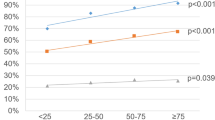
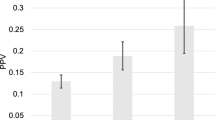
504 Chicago men and 6980 Stockholm men were included. In African American (AA) men, 51% had GG ≥ 2 PC detected, while in risk-matched Stockholm men, 34% had GG ≥ 2 PC detected (OR: 2.1, p < 0.001). There was no statistical difference in GG ≥ 2 PC detected when matching Stockholm men to non-Hispanic Caucasian men (31% vs. 24%, OR: 0.7, p = 0.30) or Hispanic Caucasian men (31% vs. 27%, OR: 1.2, p = 0.42). The AUC for the Stockholm3 test of the matched Stockholm cohorts for AA, non-Hispanic Caucasian, and Hispanic Caucasian men was 0.85, 0.89, and 0.90, respectively.
Conclusions
Using statistical techniques to simulate a multi-ethnic Chicago cohort within the STHLM3 population, we found an excess risk of GG ≥ 2 PC among AA men. Our hypothesis that the Stockholm3 may have good predictive value in a multiethnic cohort is strengthened, and that recalibration to at least AA men seems likely to be needed to obtain well-calibrated predictions.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 4 print issues and online access
269,00 € per year
only 67,25 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30.
Potts JM, Lutz M, Walker E, Modlin C, Klein E. Trends in PSA, age and prostate cancer detection among black and white men from 1990-2006 at a tertiary care center. Cancer. 2010;116:3910–5.
Dobbs RW, Malhotra NR, Abern MR, Moreira DM. Prostate cancer disparities in Hispanics by country of origin: a nationwide population-based analysis. Prostate Cancer Prostatic Dis. 2019;22:159–67.
Velasquez MC, Chinea FM, Kwon D, Prakash NS, Barboza MP, Gonzalgo ML, et al. The influence of ethnic heterogeneity on prostate cancer mortality after radical prostatectomy in Hispanic or Latino men: a population-based analysis. Urology. 2018;117:108–14.
Schroder FH, Hugosson J, Roobol MJ, Tammela TL, Zappa M, Nelen V, et al. Screening and prostate cancer mortality: results of the European Randomised Study of Screening for Prostate Cancer (ERSPC) at 13 years of follow-up. Lancet. 2014;384:2027–35.
Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol. 2017;71:353–65.
Vickers AJ, Roobol MJ, Lilja H. Screening for prostate cancer: early detection or overdetection? Annu Rev Med. 2012;63:161–70.
Ankerst DP, Hoefler J, Bock S, Goodman PJ, Vickers A, Hernandez J, et al. Prostate cancer prevention trial risk calculator 2.0 for the prediction of low- vs high-grade prostate cancer. Urology. 2014;83:1362–7.
Ankerst DP, Straubinger J, Selig K, Guerrios L, De Hoedt A, Hernandez J, et al. A contemporary prostate biopsy risk calculator based on multiple heterogeneous cohorts. Eur Urol. 2018;74:197–203.
Roobol MJ, Verbeek JFM, van der Kwast T, Kummerlin IP, Kweldam CF, van Leenders G. Improving the Rotterdam European Randomized Study of Screening for Prostate Cancer Risk Calculator for Initial Prostate Biopsy by Incorporating the 2014 International Society of Urological Pathology Gleason Grading and Cribriform growth. Eur Urol. 2017;72:45–51.
Gronberg H, Adolfsson J, Aly M, Nordstrom T, Wiklund P, Brandberg Y, et al. Prostate cancer screening in men aged 50-69 years (STHLM3): a prospective population-based diagnostic study. Lancet Oncol. 2015;16:1667–76.
Moller A, Olsson H, Gronberg H, Eklund M, Aly M, Nordstrom T. The Stockholm3 blood-test predicts clinically-significant cancer on biopsy: independent validation in a multi-center community cohort. Prostate Cancer Prostatic Dis. 2019;22:137–42.
Epstein JI, Egevad L, Amin MB, Delahunt B, Srigley JR, Humphrey PA, et al. The 2014 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma: definition of grading patterns and proposal for a new grading system. Am J Surg Pathol. 2016;40:244–52.
Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10:150–61.
Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–107.
Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9:1.
Virlogeux V, Graff RE, Hoffmann TJ, Witte JS. Replication and heritability of prostate cancer risk variants: impact of population-specific factors. Cancer Epidemiol Biomark Prev. 2015;24:938–43.
Al Olama AA, Kote-Jarai Z, Berndt SI, Conti DV, Schumacher F, Han Y, et al. A meta-analysis of 87,040 individuals identifies 23 new susceptibility loci for prostate cancer. Nat Genet. 2014;46:1103–9.
Eeles R, Goh C, Castro E, Bancroft E, Guy M, Al Olama AA, et al. The genetic epidemiology of prostate cancer and its clinical implications. Nat Rev Urol. 2014;11:18–31.
Eeles RA, Olama AA, Benlloch S, Saunders EJ, Leongamornlert DA, Tymrakiewicz M, et al. Identification of 23 new prostate cancer susceptibility loci using the iCOGS custom genotyping array. Nat Genet. 2013;45:385–91. 91e1-2.
Eeles RA, Kote-Jarai Z, Al Olama AA, Giles GG, Guy M, Severi G, et al. Identification of seven new prostate cancer susceptibility loci through a genome-wide association study. Nat Genet. 2009;41:1116–21.
Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7:e1001387.
Han Y, Signorello LB, Strom SS, Kittles RA, Rybicki BA, Stanford JL, et al. Generalizability of established prostate cancer risk variants in men of African ancestry. Int J Cancer. 2015;136:1210–7.
Rebbeck TR. Prostate cancer genetics: variation by race, ethnicity, and geography. Semin Radiat Oncol. 2017;27:3–10.
Grizzle WE, Kittles RA, Rais-Bahrami S, Shah E, Adams GW, DeGuenther MS, et al. Self-Identified African Americans and prostate cancer risk: West African genetic ancestry is associated with prostate cancer diagnosis and with higher Gleason sum on biopsy. Cancer Med. 2019;8:6915–22.
Punnen S, Freedland SJ, Polascik TJ, Loeb S, Risk MC, Savage S, et al. A multi-institutional prospective trial confirms noninvasive blood test maintains predictive value in African American men. J Urol. 2018;199:1459–63.
Schwen ZR, Tosoian JJ, Sokoll LJ, Mangold L, Humphreys E, Schaeffer EM, et al. Prostate Health Index (PHI) predicts high-stage pathology in African American men. Urology. 2016;90:136–40.
Bock CH, Powell I, Kittles RA, Hsing AW, Carpten J. Racial disparities in prostate cancer incidence, biochemical recurrence, and mortality. Prostate Cancer. 2011;2011:716178.
Porten SP, Richardson DA, Odisho AY, McAninch JW, Carroll PR, Cooperberg MR. Disproportionate presentation of high risk prostate cancer in a safety net health system. J Urol. 2010;184:1931–6.
Zeigler-Johnson CM, Tierney A, Rebbeck TR, Rundle A. Prostate cancer severity associations with neighborhood deprivation. Prostate Cancer. 2011;2011:846263.
Acknowledgements
We thank Patrice King-Lee for data collection.
Funding
We recieved funding from Stockholm County Council, Cancerfonden, Swedish Research Council.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. HG has five prostate cancer diagnostic–related patents pending, has patent applications licensed to Thermo Fisher Scientific, and might receive royalties from sales related to these patents. Dr. ME has five prostate cancer diagnostic–related patents pending. Karolinska Institutet collaborates with Thermo Fisher Scientific in developing the technology for the Stockholm3 test. The remaining authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Vigneswaran, H.T., Discacciati, A., Gann, P.H. et al. Ethnic variation in prostate cancer detection: a feasibility study for use of the Stockholm3 test in a multiethnic U.S. cohort. Prostate Cancer Prostatic Dis 24, 120–127 (2021). https://doi.org/10.1038/s41391-020-0250-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41391-020-0250-2
This article is cited by
-
Disparities in prostate cancer diagnosis and management: recognizing that disparities exist at all junctures along the prostate cancer journey
Prostate Cancer and Prostatic Diseases (2023)