Abstract
Study design
Retrospective chart audit.
Objectives
This study aimed to identify conventional routine blood testing biomarkers associated with the progression of intramedullary injured area in patients with spinal cord injury (SCI).
Setting
A spinal cord injury center in Hokkaido, Japan.
Methods
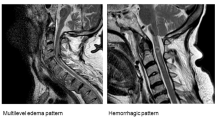
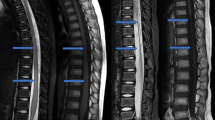
We retrospectively reviewed 71 consecutive adults with acute SCI who were admitted within 24 h after injury and diagnosed as American Spinal Injury Association Impairment Scale Grade A or B at admission. Participants were divided into the progression (P group) and no progression group (NP group) based on the change of the hyperintense signal abnormality in the spinal cord on magnetic resonance imaging from the time of admission to 4 weeks after injury. Individual characteristics and blood testing data obtained in the first 4 weeks after injury were compared between groups.
Results
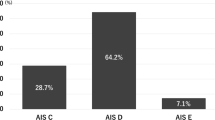
The P and NP groups were comprised of 16 and 55 participants, respectively. In univariate analyses, white blood cell (WBC) count on day 3 was significantly higher in group P than group NP (P = 0.021), as was serum C-reactive protein (CRP) level on day 3 (P = 0.015) and day 7 (P = 0.047). Multivariable analysis identified serum CRP level on day 3 as a significant independent prognostic factor for the progression of secondary SCI (OR, 1.138; 95% confidence interval, 1.01–1.28; P = 0.034).
Conclusions
Serum CRP level on day 3 after injury was a good predictor for the progression of intramedullary signal intensity change on MRI from acute to subacute stage in patients with SCI.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Tsuji O, Miura K, Okada Y, Fujiyoshi K, Mukaino M, Nagoshi N, et al. Therapeutic potential of appropriately evaluated safe-induced pluripotent stem cells for spinal cord injury. Proc Natl Acad Sci USA. 2010;107:12704–9.
Kawabata S, Takano M, Numasawa-Kuroiwa Y, Itakura G, Kobayashi Y, Nishiyama Y, et al. Grafted human iPS cell-derived oligodenrtocyte precursor cells contribute to robust remyelination of demyelinated axons after spinal cord injury. Stem Cell Rep. 2016;6:1–8.
Nagoshi N, Tsuji O, Kitamura K, Suda K, Maeda T, Yato Y, et al. Phase I/II study of intrathecal administration of recombinant human hepatocyte growth factor in patients with acute spinal cord injury: a double-blind, randomized clinical trial of safety and efficacy. J Neurotrauma. 2020;37:1752–8.
Koda M, Hanaoka H, Sato T, Fujii Y, Hanawa M, Takahashi S, et al. Study Protocol for the G-SPIRIT trial: a randomized placebo-controlled, double-blinded phase III trial of granulocyte colony-stimulating factor-mediated neuroprotection for acute spinal cord injury. BMJ Open. 2018;8:e019083.
Tetreault LA, Zhu MP, Wilson JR, Karadimas SK, Fehlings MG. The impact of Riluzole on neurobehavioral outcomes in preclinical models of traumatic and nontraumatic spinal cord injury: results from a systematic review of the literature. Glob Spine J. 2020;10:216–29.
McDonald JW, Sadowsky C. Spinal-cord injury. Lancet 2002;359:417–25.
Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26.
Profyris C, Cheema S, Zang D, Azari M, Boyle K, Petratos S. Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis. 2004;15:415–36.
Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp. 2011;71:281–99.
Allan SM, Rothwell NJ. Inflammation in central nervous system injury. Philos Trans R Soc Lond B Biol Sci. 2003;358:1669–77.
Grossman RG, Fehlings MG, Frankowski RF, Burau KD, Chow DSL, Tator C, et al. A prospective, multicenter, phase I matched-comparison group trial of safety, pharmacokinetics, and preliminary efficacy of riluzole in patients with traumatic spinal cord injury. J Neurotrauma. 2014;31:239–55.
Pouw MH, Kwon BK, Verbeek MM, Vos PE, van Kampen A, Fisher CG, et al. Structural biomarkers in the cerebrospinal fluid within 24 h after a traumatic spinal cord injury: a descriptive analysis of 16 subjects. Spinal Cord. 2014;52:428–33.
Kwon BK, Streijger F, Fallah N, Noonan VK, Belanger LM, Ritchie L, et al. Cerebrospinal fluid biomarkers to stratify injury severity and predict outcome in human traumatic spinal cord injury. J Neurotrauma. 2017;34:567–80.
Biglari B, Swing T, Child C, Büchler A, Westhauser F, Bruckner T, et al. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: the serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord. 2015;53:510–14.
Hachisuka S, Kamei N, Ujigo S, Miyaki S, Yasunaga Y, Ochi M. Circulating microRNAs as biomarkers for evaluating the severity of acute spinal cord injury. Spinal Cord. 2014;52:596–600.
Davies AL, Hayes KC, Dekaban GA. Clinical correlates of elevated serum concentrations of cytokines and autoantibodies in patients with spinal cord injury. Arch Phys Med Rehabil. 2007;88:1384–93.
Katoh S, Ikata T, Tsubo M, Hamada Y, el Masry WS. Possible implication of leukocytes in secondary pathological changes after spinal cord injury. Injury 1997;28:215–7.
Tong B, Jutzeler CR, Cragg JJ, Grassner L, Schwab JM, Casha S, et al. Serum albumin predicts long-term neurological outcomes after acute spinal cord injury. Neurorehabil Neural Repair. 2018;32:7–17.
Kirshblum SC, Burns SP, Biering-Sorensen F, Donovan W, Graves DE, Jha A, et al. International standards for neurological classification of spinal cord inury (revised 2011). J Spinal Cord Med. 2011;34:535–46.
Rutges J, Kwon BK, Heran M, Ailon T, Street JT, Dvorak MF. A prospective serial MRI study following acute traumatic cervical spinal cord injury. Eur Spine J. 2017;26:2324–32.
Shibata H, Haga H, Ueno M, Nagai H, Yasumura S, Koyano W. Longitudinal changes of serum albumin in elderly people living in the community. Age Ageing. 1991;20:417–20.
Greenblatt DJ. Reduced serum albumin concentration in the elderly: a report from the Boston collaborative drug surveillance program. J AM Geriatr Soc. 1979;27:20–2.
Goldstein RL, Walia P, Teylan M, Lazzari AA, Tun CG, Hart JE, et al. Clinical factors associated with c-reactive protein in chronic spinal cord injury. Spinal Cord. 2017;55:1088–95.
Nakamura M, Houghtling RA, MacArthur L, Bayer BM, Bregman BS. Differences in cytokine gene expression profile between acute and secondary injury in adult rat spinal cord. Exp Neurol. 2003;184:313–25.
Ahuja CS, Nori S, Tetreault L, Wilson J, Kwon B, Harrop J, et al. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 2017;80:S9–S22.
Bartanusz V, Jezova D, Alajajian B, Digicaylioglu M. The blood-spinal cord barrier: morphology and clinical implications. Ann Neurol. 2011;70:194–206.
Nishikawa T, Hagihara K, Serada S, Isobe T, Matsumura A, Song J, et al. Transcriptional complex formation of c-Fos, STAT3, and Hepatocyte NF-1 alpha is essential for cytokine-driven c-reactive protein gene expression. J Immunol. 2008;180:3492–501.
Biglari B, Swing T, Child C, Büchler A, Westhauser F, Bruckner T, et al. A pilot study on temporal changes in IL-1β and TNF-α serum levels after spinal cord injury: the serum level of TNF-α in acute SCI patients as a possible marker for neurological remission. Spinal Cord. 2015;53:510–4.
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–12.
Acknowledgements
We are grateful to all individuals who participated in this study. No financial assistance was received in support of this study.
Author information
Authors and Affiliations
Contributions
MO collected and interpreted the data and the MRI and wrote the initial draft of the manuscript. SK designed the study, collected and interpreted the data and the MRI, and critically reviewed the manuscript. TK and SMH collected the data and reviewed the manuscript. MK, AM, MM, MN, MT and NI reviewed the submitted version of the manuscript. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This study was approved by the ethics committee of our institution.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ozaki, M., Suda, K., Konomi, T. et al. Serum C-reactive protein is an early, simple and inexpensive prognostic marker for the progression of intramedullary lesion on magnetic resonance imaging from acute to subacute stage in patients with spinal cord injury. Spinal Cord 59, 1155–1161 (2021). https://doi.org/10.1038/s41393-021-00640-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41393-021-00640-6
This article is cited by
-
Serum glucose/potassium ratio as a clinical risk factor for predicting the severity and prognosis of acute traumatic spinal cord injury
BMC Musculoskeletal Disorders (2023)