Abstract
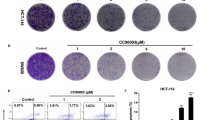
Our previous study showed that TP53-induced glycolysis and apoptosis regulator (TIGAR) regulated ROS, autophagy, and apoptosis in response to hypoxia and chemotherapeutic drugs. Aescin, a triterpene saponin, exerts anticancer effects and increases ROS levels. The ROS is a key upstream signaling to activate autophagy. Whether there is a crosstalk between TIGAR and aescin in regulating ROS, autophagy, and apoptosis is unknown. In this study, we found that aescin inhibited cell viability and colony formation, and induced DNA damage, cell cycle arrest, and apoptosis in cancer cell lines HCT-116 and HCT-8 cells. Concurrently, aescin increased the expression of TIGAR, ROS levels, and autophagy activation. Knockdown of TIGAR enhanced the anticancer effects of aescin in vitro and in vivo, whereas overexpression of TIGAR or replenishing TIGAR downstream products, NADPH and ribose, attenuated aescin-induced apoptosis. Furthermore, aescin-induced ROS elevation and autophagy activation were further strengthened by TIGAR knockdown in HCT-116 cells. However, autophagy inhibition by knockdown of autophagy-related gene ATG5 or 3-methyladenine (3-MA) exaggerated aescin-induced apoptosis when TIGAR was knocked down. In conclusion, TIGAR plays a dual role in determining cancer cell fate via inhibiting both apoptosis and autophagy in response to aescin, which indicated that inhibition of TIGAR and/or autophagy may be a junctional therapeutic target in treatment of cancers with aescin.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30.
Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin. 2017;67:177–93.
Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26.
Fearon ER. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507.
Markowitz SD, Bertagnolli MM. Molecular origins of cancer: molecular basis of colorectal cancer. N Engl J Med. 2009;361:2449–60.
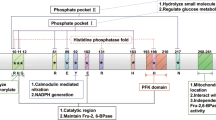
Bensaad K, Tsuruta A, Selak MA, Vidal MN, Nakano K, Bartrons R, et al. TIGAR, a p53-inducible regulator ofglycolysis and apoptosis. Cell. 2006;126:107–20.
Bensaad K, Cheung EC, Vousden KH. Modulation of intracellular ROS levels by TIGAR controls autophagy. EMBO J. 2009;28:3015–26.
Won KY, Lim SJ, Kim GY, Kim YW, Han SA, Song JY, et al. Regulatory role ofp53 in cancer metabolism via SCO2 and TIGAR in human breast cancer. Hum Pathol. 2012;43:221–8.
Wanka C, Steinbach JP, Rieger J. Tp53-induced glycolysis and apoptosis regulator (TIGAR) protects glioma cells from starvation-induced cell death by up- regulating respiration and improving cellular redox homeostasis. J Biol Chem. 2012;287:33436–46.
Qian S, Li J, Hong M, Zhu Y, Zhao H, Xie Y, et al. TIGAR cooperated with glycolysis to inhibit the apoptosis of leukemia cells and associated with poor prognosis in patients with cytogeneti-cally normal acute myeloid leukemia. J Hematol Oncol. 2016;9:128.
Wong EY, Wong SC, Chan CM, Lam EK, Ho LY, Lau CP, et al. TP53-induced glycolysis and apoptosis regulator promotes proliferation and invasiveness of nasopharyngeal carcinoma cells. Oncol Lett. 2015;9:569–74.
Al-Khayal K, Abdulla M, Al-Obeed O, Al Kattan W, Zubaidi A, Vaali-Mohammed MA, et al. Identification of the TP53-induced glycolysis and apoptosis regulator in various stages of colorectal cancer patients. Oncol Rep. 2016;35:1281–6.
Cheung EC, Athineos D, Lee P, Ridgway RA, Lambie W, Nixon C, et al. TIGAR is required for efficient intestinal regeneration and tumorigenesis. Dev Cell. 2013;25:463–77.
Xie JM, Li B, Yu HP, Gao QG, Li W, Wu HR, et al. TIGAR has a dual role in cancer cell survival through regulating apoptosis and autophagy. Cancer Res. 2014;74:5127–38.
Yu HP, Xie JM, Li B, Sun YH, Gao QG, Ding ZH, et al. TIGAR regulates DNA damage and repair through pentosepho-sphate pathway and Cdk5-ATM pathway. Sci Rep. 2015;5:9853.
Patlolla JM, Raju J, Swamy MV, Rao CV. Beta-escin inhibits colonic aberrant crypt foci formation in rats and regulates the cell cycle growthbyinducingp21(waf1/cip1) in colon cancer cells. Mol Cancer Ther. 2006;5:1459–66.
Tan SM, Li F, Rajendran P, Kumar AP, Hui KM, Sethi G. Identification of beta-escin as a novel inhibitor of signal transducer and activator oftranscription 3/Janus-activated kinase 2 signaling pathway that suppresses proliferation and induces apoptosis in human hepatocellular carcinoma cells. J Pharmacol Exp Ther. 2010;334:285–93.
Ji DB, Xu B, Liu JT, Ran FX, Cui J. R. beta-Escin sodium inhibits inducible nitric oxide synthase expression via downregulation of the JAK/STAT pathway in A549 cells. Mol Carcinog. 2011;50:945–60.
Harikumar KB, Sung B, Pandey MK, Guha S, Krishnan S, Aggarwal BB. Escin, a pentacyclic triterpene, chemosensitizes human tumor cells through inhibition of nuclear factor-kappaB signaling pathway. Mol Pharmacol. 2010;77:818–27.
Ming ZJ, Hu Y, Qiu YH, Cao L, Zhang XG. Synergistic effects of beta-aescin and 5-fluorouracil in human hepatocellular carcinoma SMMC-7721 cells. Phytomedicine. 2010;17:575–80.
Wang YW, Wang SJ, Zhou YN, Pan SH, Sun B. Escin augments the efficacy of gemcitabine through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in pancreatic cancer both in vitro and in vivo. J Cancer Res Clin Oncol. 2012;138:785–97.
Huang GL, Shen DY, Cai CF, Zhang QY, Ren HY, Chen QX. Beta-escin reverses multidrug resistance through inhibition of the GSK3beta/beta-catenin pathway in cholangiocarcinoma. World J Gastroenterol. 2015;21:1148–57.
Lee HS, Hong JE, Kim EJ, Kim SH. Escin suppresses migration and invasion involving the alteration of CXCL16/CXCR6 axis in human gastric adenocarcinoma AGS cells. Nutr Cancer. 2014;66:938–45.
Wang Y, Xu X, Zhao P, Tong B, Wei Z, Dai Y. Escin Ia suppresses the metastasis of triple-negative breast cancer by inhibiting epithelial-mesenchymal transition via down-regulating LOXL2 expression. Oncotarget. 2016;7:23684–99.
Mojzisova G, Kello M, Pilatova M, Tomeckova V, Vaskova J, Vasko L, et al. Antiproliferative effect of beta-escin - an in vitro study. Acta Biochim Pol. 2016;63:79–87.
Kroemer G, Marino G, Levine B. Autophagy and the integrated stress response. Mol Cell. 2010;40:280–93.
Fang LM, Li B, Guan JJ, Xu HD, Shen GH, Gao QG, et al. Transcription factor EB is involved in autophagy-mediated chemoresistance to doxorubicin in human cancer cells. Acta Pharmacol Sin. 2017;38:1305–16.
Qian L, Liu Y, Xu Y, Ji W, Wu Q, Liu Y, et al. Matrine derivative WM130 inhibits hepatocellular carcinoma by suppressing EGFR/ERK/MMP-2 and PTEN/AKT signaling pathways. Cancer Lett. 2015;368:126–34.
Roos WP, Kaina B. DNA damage-induced cell death by apoptosis. Trends Mol Med. 2006;12:440–50.
Lee P, Hock AK, Vousden KH, Cheung EC. p53- and p73-independent activation of TIGAR expression in vivo. Cell Death Dis. 2015;6:e1842.
Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/ lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1982;79:1889–92.
Cheung EC, Ludwig RL, Vousden KH. Mitochondrial localization of TIGAR under hypoxia stimulates HK2 and lowers ROS and cell death. Proc Natl Acad Sci U S A. 2012;109:20491–6.
Zhang H, Gu C, Yu J, Wang Z, Yuan X, Yang L, et al. Radiosensitization of glioma cells by TP53-induced glycolysis and apoptosis regulator knockdown is dependent on thioredoxin-1 nuclear translocation. Free Radic Biol Med. 2014;69:239–48.
Jackson SP, Bartek J. The DNA-damage response in human biology and disease. Nature. 2009;461:1071–8.
Mizushima N, Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147:728–41.
Zhang L, Wang K, Lei Y, Li Q, Nice EC, Huang C. Redox signaling: potential arbitrator of autophagy and apoptosis in therapeutic response. Free Radic Biol Med. 2015;89:452–65.
White E. Deconvoluting the context-dependent role for autophagy in cancer. Nat Rev Cancer. 2012;12:401–10.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81770483 and 81602613), Jiangsu Provincial Commission of Health and Family Planning (No. YG201402 and YG201503), Jiangsu Provincial Medical Youth Talent (No. QNRC2016249), Jiangsu Provincial Science and Technology office (No. BL2014043), Suzhou Science and Technology Bureau (No. SYSD2013041, SYSD2016044, SYSD2017041, and SYS201788), Suzhou Health and Family Planning Commission Program (No. LCZX201504), Wujiang District Science and Technology Bureau (No. WS201301), and Wujiang District Commission of Health and Family Planning (No. WWK201607 and WWK201609).
Author information
Authors and Affiliations
Contributions
All listed authors contributed to the idea generation, design, and completion of this study. B.L., Z.W., and J.-M.X. contributed equally to the idea generation, experimental work, and manuscript preparation; G.W., L.-Q.Q., X.-M.G., and X.-P.S. contributed to the experimental work and manuscript preparation; X.-Q.L., Q.-G.G., G.-H.S., and Z.-H.Q. guided the idea generation, experimental work, and manuscript preparation. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Li, B., Wang, Z., Xie, Jm. et al. TIGAR knockdown enhanced the anticancer effect of aescin via regulating autophagy and apoptosis in colorectal cancer cells. Acta Pharmacol Sin 40, 111–121 (2019). https://doi.org/10.1038/s41401-018-0001-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-018-0001-2
Keywords
This article is cited by
-
TIGAR coordinates senescence-associated secretory phenotype via lysosome repositioning and α-tubulin deacetylation
Experimental & Molecular Medicine (2024)
-
Role of Escin in breast cancer therapy: potential mechanism for inducing ferroptosis and synergistic antitumor activity with cisplatin
Apoptosis (2023)
-
MiR-652-5p elevated glycolysis level by targeting TIGAR in T-cell acute lymphoblastic leukemia
Cell Death & Disease (2022)
-
Online thickness prediction of hot-rolled strip based on ISSA-OSELM
International Journal on Interactive Design and Manufacturing (IJIDeM) (2022)
-
Smart microgels as drug delivery vehicles for the natural drug aescin: uptake, release and interactions
Colloid and Polymer Science (2020)