Abstract
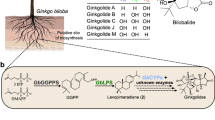
Terpene lactones are a class of bioactive constituents of standardized preparations of Ginkgo biloba leaf extract, extensively used as add-on therapies in patients with ischemic cardiovascular and cerebrovascular diseases. This investigation evaluated human pharmacokinetics of ginkgo terpene lactones and impact of their carboxylation in blood. Human subjects received oral YinXing-TongZhi tablet or intravenous ShuXueNing, two standardized ginkgo preparations. Their plasma protein-binding and platelet-activating factor antagonistic activity were assessed in vitro. Their carboxylation was assessed in phosphate-buffered saline (pH 7.4) and in human plasma. After dosing YinXing-TongZhi tablet, ginkgolides A and B and bilobalide exhibited significantly higher systemic exposure levels than ginkgolides C and J; after dosing ShuXueNing, ginkgolides A, B, C, and J exhibited high exposure levels. The compounds’ unbound fractions in plasma were 45–92%. Apparent oral bioavailability of ginkgolides A and B was mostly >100%, while that of ginkgolides C and J was 6–15%. Bilobalide’s bioavailability was probably high but lower than that of ginkgolides A/B. Terminal half-lives of ginkgolides A, B, and C (4–7 h) after dosing ShuXueNing were shorter than their respective values (6–13 h) after dosing YinXing-TongZhi tablet. Half-life of bilobalide after dosing the tablet was around 5 h. Terpene lactones were roughly evenly distributed in various body fluids and tissues; glomerular-filtration-based renal excretion was the predominant elimination route for the ginkgolides and a major route for bilobalide. Terpene lactones circulated as trilactones and monocarboxylates. Carboxylation reduced platelet-activating factor antagonistic activity of ginkgolides A, B, and C. Ginkgolide J, bilobalide, and ginkgo flavonoids exhibited no such bioactivity. Collectively, differences in terpene lactones’ exposure between the two preparations and influence of their carboxylation in blood should be considered in investigating the relative contributions of terpene lactones to ginkgo preparations’ therapeutic effects. The results here will inform rational clinical use of ginkgo preparations.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Drieu K, Jaggy H. History, development and constituents of EGb761. In: van Beek TA, editor. Ginkgo biloba. Armsterdam: Harwood Academic Publishers; 2000. p. 267–77.
DeFeudis FV. A brief history of EGb 761 and its therapeutic uses. Pharmacopsychiatry. 2003;36:S2–7.
Strømgaard K, Nakanishi K. Chemistry and biology of terpene trilactones from Ginkgo biloba. Angew Chem Int Ed. 2004;43:1640–58.
Blumenthal M. The ABC clinical guide to herbs. New York: Thieme; 2003. pp. 185–200.
van Beek TA, Montoro P. Chemical analysis and quality control of Ginkgo biloba leaves, extracts, and phytopharmaceuticals. J Chromatogr A. 2009;1216:2002–32.
Gu J-H, Ge J-B, Li M, Wu F, Zhang W, Qin Z-H. Inhibition of NF-κB activation is associated with anti-inflammatory and anti-apoptotic effects of Ginkgolide B in a mouse model of cerebral ischemia/reperfusion injury. Eur J Pharm Sci. 2012;47:652–60.
Zhao Q-P, Gao C-Y, Cui Z-F. Ginkgolide A reduces inflammatory response in high-glucose-stimulated human umbilical vein endothelial cells through STAT3-mediated pathway. Int Immunopharmacol. 2015;25:242–8.
Zhang S, Chen B-D, Wu W, Bao L, Qi R-M. Ginkgolide B reduces inflammatory protein expression in oxidized low-density lipoprotein-stimulated human vascular endothelial cells. J Cardiovasc Pharmacol. 2011;57:721–7.
Zhou W, Chai H, Courson A, Lin PH, Lumsden AB, Yao Q-Z, et al. Ginkgolide A attenuates homocysteine-induced endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2006;44:853–62.
Maerz S, Liu C-H, Guo W, Zhu Y-Z. Anti-ischaemic effects of bilobalide on neonatal rat cardiomyocytes and the involvement of the platelet-activating factor receptor. Biosci Rep. 2011;31:439–47.
Cho HJ, Nam KS. Inhibitory effect of ginkgolide B on platelet aggregation in a cAMP- and cGMP-dependent manner by activated MMP-9. J Biochem Mol Biol. 2007;40:678–83.
Cheung F, Siow YL, O K. Inhibition by ginkgolides and bilobalide of the production of nitric oxide in macrophages (THP-1) but not in endothelial cells (HUVEC). Biochem Pharmacol. 2001;61:503–10.
Priyanka A, Nisha VM, Anusree SS, Raghu KG. Bilobalide attenuates hypoxia induced oxidative stress, inflammation, and mitochondrial dysfunctions in 3T3-L1 adipocytes via its antioxidant potential. Free Radic Res. 2014;48:1206–17.
Lachachi H, Plantavid M, Simon MF, Chap H, Braquet P, Douste-Blazy L. Inhibition of transmembrane movement and metabolism of platelet activating factor (PAF-acether) by a specific antagonist, BN 52021. Biochem Biophys Res Commun. 1985;132:460–6.
Lamant V, Mauco G, Braquet P, Chap H, Douste-Blazy L. Inhibition of the metabolism of platelet activating factor (PAF-acether) by three specific antagonists from Ginkgo biloba. Biochem Pharmacol. 1987;36:2749–52.
Montrucchio G, Alloatti G, Camussi G. Role of platelet-activating factor in cardiovascular pathophysiology. Physiol Rev. 2000;80:1669–99.
Yoo H, Ku SK, Baek YD, Bae JS. Anti-inflammatory effects of rutin on HMGB1-induced inflammatory responses in vitro and in vivo. Inflamm Res. 2014;63:197–206.
Choi JS, Kang SW, Li J, Kim JL, Bae JY, Kim DS, et al. Blockade of oxidized LDL-triggered endothelial apoptosis by quercetin and rutin through differential signaling pathways involving JAK2. J Agric Food Chem. 2009;57:2079–86.
Afana’ev IB, Dorozhko AI, Brodskii AV, Kostyuk VA, Potapovitch AI. Chelating and free radiacal scavenging mechanisms of inhibitory action of rutin and quercetin in lipid peroxidation. Biochem Pharmacol. 1989;38:1763–9.
Li L, Zhao Y-S, Du F-F, Yang J-L, Xu F, Niu W, et al. Intestinal absorption and presystemic elimination of various chemical constituents present in GBE50 extract, a standardized extract of Ginkgo biloba leaves. Curr Drug Metab. 2012;13:494–509.
Chen F, Li L, Xu F, Sun Y, Du F-F, Ma X-T, et al. Systemic and cerebral exposure to and pharmacokinetics of flavonols and terpene lactones after dosing standardized Ginkgo biloba leaf extracts to rats via different routes of administration. Br J Pharmacol. 2013;170:440–57.
Sousa T, Paterson R, Moore V, Carlsson A, Abrahamsson B, Basit AW. The gastrointestinal microbiota as a site for the biotransformation of drugs. Int J Pharm. 2008;363:1–25.
Mahmood I, Sahajwalla C. Interspecies scaling of biliary excreted drugs. J Pharm Sci. 2002;91:1908–14.
Mauri P, Simonetti P, Gardana C, Minoggio M, Morazzoni P, Bombardelli E, et al. Liquid chromatography/atmospheric pressure chemical ionization mass spectrometry of terpene lactones in plasma of volunteers dosed with Ginkgo biloba L. extracts. Rapid Commun Mass Spectrom. 2001;15:929–34.
Woelkart K, Feizlmayr E, Dittrich P, Beubler E, Pinl F, Suter A, et al. Pharmacokinetics of bilobalide, ginkgolide A and B after administration of three different Ginkgo biloba L. preparations in humans. Phytother Res. 2010;24:445–50.
Drago F, Floriddia ML, Cro M, Giuffrida S. Pharmacokinetics and bioavailability of a Ginkgo biloba extract. J Ocul Pharmacol Ther. 2002;18:197–202.
Wang FM, Yao TW, Zeng S. Determination of quercetin and kaempferol in human urine after orally administered tablet of Ginkgo biloba extract by HPLC. J Pharm Biomed Anal. 2003;33:317–21.
Ude C, Schubert-Zsilavecz M, Wurglics M. Ginkgo biloba extracts: a review of the pharmacokinetics of the active ingredients. Clin Pharmacokinet. 2013;52:727–49.
Maclennan KM, Darlington CL, Smith PF. The CNS effects of Ginkgo biloba extracts and ginkgolide B. Prog Neurobiol. 2002;67:235–57.
Zekri O, Boudeville P, Genay P, Perly B, Braquet P, Jouenne P, et al. Ionization constants of ginkgolide B in aqueous solution. Anal Chem. 1996;68:2598–604.
Lan K, Li X-J, Du G, Xu L. Characterizations of the hydrolyzed products of ginkgolide A and ginkgolide B by liquid chromatography coupled with mass spectrometry. J Pharm Biomed Anal. 2016;118:113–22.
Draganov DI, La DuBN. Pharmacogenetics of paraoxonases: a brief review. Naunyn Schmiede Arch Pharmacol. 2004;369:78–88.
Wang D-L, Peng D-Y, Tao X-H, Cao Y, Chen W-D, Liang Y, et al. The pharmacokinetics and conversion of the lactone to the carboxylate forms of ginkgolide B in rat plasma. J Asian Nat Prod Res. 2013;15:337–43.
Liu X-G, Qi L-W, Fan Z-Y, Dong X, Guo R-Z, Lou F-C, et al. Accurate analysis of ginkgolides and their hydrolyzed metabolites by analytical supercritical fluid chromatography hybrid tandem mass spectrometry. J Chromatogr A. 2015;1388:251–8.
Li X-J, Wang Y-Q, Yang J, Fan X, Wang L, Yang K, et al. Semi-quantitative determination of monocarboxylate forms of ginkgolide B in plasma by UPLC-MS. Anal Bioanal Chem. 2015;407:4121–9.
Guo B, Li C, Wang G-J, Chen L-S. Rapid and direct measurement of free concentrations of highly protein-bound fluoxetine and its metabolite norfluoxetine in plasma. Rapid Commun Mass Spectrom. 2006;20:39–47.
Davies B, Morris T. Physiological parameters in laboratory animals and humans. Pharm Res. 1993;10:1093–5.
Jia W-W, Du F-F, Liu X-W, Jiang R-R, Xu F, Yang J-L, et al. Renal tubular secretion of tanshinol: molecular mechanisms, impact on its systemic exposure, and propensity for dose-related nephrotoxicity and for renal herb-drug interactions. Drug Metab Dispos. 2015;43:669–78.
Zhao Y, Sun Y, Li C. Simultaneous determination of ginkgo flavonoids and terpenoids in plasma: ammonium formate in LC mobile phase enhancing electrospray ionization efficiency and capacity. J Am Soc Mass Spectrom. 2008;19:445–9.
Feng B, LaPerle JL, Chang G, Varma MV. Renal clearance in drug discovery and development: molecular descriptors, drug transporters and disease state. Expert Opin Drug Metab Toxicol. 2010;6:939–52.
Varma MV, Feng B, Obach RS, Troutman MD, Chupka J, Miller HR, et al. Physicochemical determinants of human renal clearence. J Med Chem. 2009;52:4844–52.
Lv H, Wang G-J, Wu X-L, Xie L, Huang C-R, Li H, et al. Transport characteristics of ginkgolide B by Caco-2 cells and examination of ginkgolide B oral absorption potential using rat in situ intestinal loop method. Int J Pharm. 2008;351:31–5.
Yang X-N, Gandhi YA, Duignan DR, Morris ME. Prediction of biliary excretion in rats and humans using molecular weight and quantitative structure-pharmacokinetic relationships. AAPS J. 2009;11:511–25.
Gao Q, Zhang Y-F, Wo S, Zuo Z. Hydrolysis is the dominating in vivo metabolism pathway for arctigenin: identification of novel metabolites of arctigenin by LC/MS/MS after oral administration in rats. Planta Med. 2013;79:471–9.
Hioki T, Fukami T, Nakajima M, Yokoi T. Human paraoxonase 1 is the enzyme responsible for pilocarpine hydrolysis. Drug Metab Dispos. 2011;39:1345–52.
Draganov DI, Teiber JF, Speelman A, Osawa Y, Sunahara R, La Du BN. Human paraoxonases (PON1, PON2, and PON3) are lactonases with overlapping and distinct substrate specificities. J Lipid Res. 2005;46:1239–47.
Pla A, Rodrigo L, Hernández AF, Gil F, Lopez O. Effect of metal ions and calcium on purified PON1 and PON3 from rat liver. Chem Biol Interact. 2007;167:63–70.
Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–92.
Acknowledgements
This work was funded by grants from the Science & Technology Commission of Shanghai Municipality (13DZ1970200), the National Science & Technology Major Project of China “Key New Drug Creation and Manufacturing Program” (2009ZX09304-002 and 2017ZX09301012006), the National Science Foundation of China for Distinguished Young Scholars (30925044) and the Strategic Priority Research Program of the Chinese Academy of Sciences (XDA12050306).
Author contributions
Participated in research design: CL, LL, Y-hH. Conducted experiments: LL, X-wL, J-lY, WN, W-wJ, QW, X-nD, F-qW, F-fD, C-cZ, Y-fL, FX. Performed data analysis: CL, LL, QG, X-wL. Wrote or contributed to the writing of the manuscript: CL, LL, OEO, X-wL.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Liu, Xw., Yang, Jl., Niu, W. et al. Human pharmacokinetics of ginkgo terpene lactones and impact of carboxylation in blood on their platelet-activating factor antagonistic activity. Acta Pharmacol Sin 39, 1935–1946 (2018). https://doi.org/10.1038/s41401-018-0086-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-018-0086-7
Keywords
This article is cited by
-
Investigating the therapeutic potential of terpene metabolites in hot-natured herbal medicines and their mechanistic impact on circulatory disorders
Phytochemistry Reviews (2025)
-
Multi-compound and drug-combination pharmacokinetic research on Chinese herbal medicines
Acta Pharmacologica Sinica (2022)
-
Pharmacokinetics-based identification of pseudoaldosterogenic compounds originating from Glycyrrhiza uralensis roots (Gancao) after dosing LianhuaQingwen capsule
Acta Pharmacologica Sinica (2021)
-
Multiple circulating alkaloids and saponins from intravenous Kang-Ai injection inhibit human cytochrome P450 and UDP-glucuronosyltransferase isozymes: potential drug–drug interactions
Chinese Medicine (2020)
-
The therapeutic potential of bilobalide on experimental autoimmune encephalomyelitis (EAE) mice
Metabolic Brain Disease (2020)