Abstract
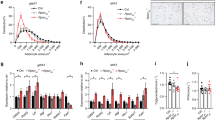
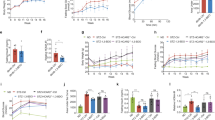
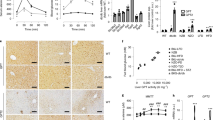
Osteocalcin, expressed in osteoblasts of the bone marrow, undergoes post-translational carboxylation and deposits in mineralized bone matrix. A portion of osteocalcin remains uncarboxylated (uncarboxylated osteocalcin, GluOC) that is released into blood where it functions as a hormone to regulate insulin secretion and insulin sensitivity. As insulin resistance is closely associated with metabolic syndrome, this study is aimed to elucidate how GluOC regulates glucose and lipid metabolism in KKAy mice, an animal model displaying obese, hyperglycemia, hyperinsulinemia, insulin resistance, and hepatic steatosis. GluOC (3, 30 ng/g per day, ig) was orally administered to female KKAy mice for 4 weeks. Whole-body insulin sensitivity, glucose metabolism, hepatic steatosis, dyslipidemia were examined using routine laboratory assays. We found that GluOC administration significantly enhanced insulin sensitivity in KKAy mice by activating hepatic IRβ/PI3K/Akt pathway and elevated the whole-body insulin sensitivity with decreased FPI and HOMA-IR index. Furthermore, GluOC administration alleviated hyperglycemia through suppressing gluconeogenesis and promoting glycogen synthesis in KKAy mice and in cultured hepatocytes in vitro. Moreover, GluOC administration dose-dependently ameliorated dyslipidemia and attenuated hepatic steatosis in KKAy mice by inhibiting hepatic de novo lipogenesis and promoting fatty-acid β-oxidation. These results demonstrate that GluOC effectively enhances hepatic insulin sensitivity, improves hyperglycemia and ameliorates hepatic steatosis in KKAy mice, suggesting that GluOC could be a promising drug candidate for treating metabolic syndrome.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Crespo M, Lappe S, Feldstein AE, Alkhouri N. Similarities and differences between pediatric and adult nonalcoholic fatty liver disease. Metabolism. 2016;65:1161–71.
Machado M, Cortez-Pinto H. Non-alcoholic steatohepatitis and metabolic syndrome. Curr Opin Clin Nutr Metab Care. 2006;9:637–42.
Asrih M, Jornayvaz FR. Metabolic syndrome and nonalcoholic fatty liver disease: Is insulin resistance the link? Mol Cell Endocrinol. 2015;418(Pt 1):55–65.
Alam S, Mustafa G, Alam M, Ahmad N. Insulin resistance in development and progression of nonalcoholic fatty liver disease. World J Gastrointest Pathophysiol. 2016;7:211–7.
Tanaka N, Kimura T, Fujimori N, Nagaya T, Komatsu M, Tanaka E. Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J Gastroenterol. 2019;25:163–77.
Yki-Jarvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol. 2014;2:901–10.
Basaranoglu M. Understanding mechanisms of the pathogenesis of nonalcoholic fatty liver disease. World J Gastroenterol. 2010;16:2223.
Takamura T, Misu H, Ota T, Kaneko S. Fatty liver as a consequence and cause of insulin resistance: lessons from type 2 diabetic liver. Endocr J. 2012;59:745–63.
Hong F, Xu P, Zhai Y. The opportunities and challenges of peroxisome proliferator-activated receptors ligands in clinical drug discovery and development. Int J Mol Sci. 2018;19:2189.
Tang Y, Zhang X, Chen Z, Yin W, Nan G, Tian J, et al. Novel benzamido derivatives as PTP1B inhibitors with anti-hyperglycemic and lipid-lowering efficacy. Acta Pharm Sin B. 2018;8:919–32.
Zoch ML, Clemens TL, Riddle RC. New insights into the biology of osteocalcin. Bone. 2016;82:42–9.
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130:456–69.
Patti A, Gennari L, Merlotti D, Dotta F, Nuti R. Endocrine actions of osteocalcin. Int J Endocrinol. 2013;2013:846480.
Pi M, Chen L, Huang MZ, Zhu W, Ringhofer B, Luo J, et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS One. 2008;3:e3858.
Ferron M, Hinoi E, Karsenty G, Ducy P. Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci U S A. 2008;105:5266–70.
Ferron M, McKee MD, Levine RL, Ducy P, Karsenty G. Intermittent injections of osteocalcin improve glucose metabolism and prevent type 2 diabetes in mice. Bone. 2012;50:568–75.
Mizokami A, Yasutake Y, Higashi S, Kawakubo-Yasukochi T, Chishaki S, Takahashi I, et al. Oral administration of osteocalcin improves glucose utilization by stimulating glucagon-like peptide-1 secretion. Bone. 2014;69:68–79.
Guedes JAC, Esteves JV, Morais MR, Zorn TM, Furuya DT. Osteocalcin improves insulin resistance and inflammation in obese mice: Participation of white adipose tissue and bone. Bone. 2018;115:68–82.
Gupte AA, Sabek OM, Fraga D, Minze LJ, Nishimoto SK, Liu JZ, et al. Osteocalcin protects against nonalcoholic steatohepatitis in a mouse model of metabolic syndrome. Endocrinology. 2014;155:4697–705.
Yamamoto T, Yamaguchi H, Miki H, Shimada M, Nakada Y, Ogino M, et al. Coenzyme a: diacylglycerol acyltransferase 1 inhibitor ameliorates obesity, liver steatosis, and lipid metabolism abnormality in KKAy mice fed high-fat or high-carbohydrate diets. Eur J Pharmacol. 2010;640:243–9.
Fu C, Zhang X, Ye F, Yang J. High insulin levels in KK-Ay diabetic mice cause increased cortical bone mass and impaired trabecular micro-structure. Int J Mol Sci. 2015;16:8213–26.
Han TS, Sattar N, Williams K, Gonzalez-Villalpando C, Lean ME, Haffner SM. Prospective study of C-reactive protein in relation to the development of diabetes and metabolic syndrome in the Mexico City Diabetes Study. Diabetes Care. 2002;25:2016–21.
Shi QZ, Wang LW, Zhang W, Gong ZJ. Betaine inhibits toll-like receptor 4 expression in rats with ethanol-induced liver injury. World J Gastroenterol. 2010;16:897–903.
Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–7.
Ma YM, Tao RY, Liu Q, Li J, Tian JY, Zhang XL, et al. PTP1B inhibitor improves both insulin resistance and lipid abnormalities in vivo and in vitro. Mol Cell Biochem. 2011;357:65–72.
Liu HY, Collins QF, Xiong Y, Moukdar F, Lupo EG Jr., Liu Z, et al. Prolonged treatment of primary hepatocytes with oleate induces insulin resistance through p38 mitogen-activated protein kinase. J Biol Chem. 2007;282:14205–12.
Wisniewski JR, Zougman A, Nagaraj N, Mann M. Universal sample preparation method for proteome analysis. Nat Methods. 2009;6:359–62.
Tyanova S, Temu T, Carlson A, Sinitcyn P, Mann M, Cox J. Visualization of LC-MS/MS proteomics data in MaxQuant. Proteomics. 2015;15:1453–6.
Biddinger SB, Kahn CR. From mice to men: insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–58.
White MF. Insulin signaling in health and disease. Science. 2003;302:1710–1.
Gross DN, van den Heuvel AP, Birnbaum MJ. The role of FoxO in the regulation of metabolism. Oncogene. 2008;27:2320–36.
Titchenell PM, Quinn WJ, Lu M, Chu Q, Lu W, Li C, et al. Direct hepatocyte insulin signaling is required for lipogenesis but is dispensable for the suppression of glucose production. Cell Metab. 2016;23:1154–66.
Qu S, Altomonte J, Perdomo G, He J, Fan Y, Kamagate A, et al. Aberrant forkhead box O1 function is associated with impaired hepatic metabolism. Endocrinology. 2006;147:5641–52.
Shepherd PR, Withers DJ, Siddle K. Phosphoinositide 3-kinase: the key switch mechanism in insulin signalling. Biochem J. 1998;333(Pt 3):471–90.
Gruben N, Shiri-Sverdlov R, Koonen DP, Hofker MH. Nonalcoholic fatty liver disease: a main driver of insulin resistance or a dangerous liaison? Biochim Biophys Acta. 2014;1842:2329–43.
Bugianesi E, Moscatiello S, Ciaravella MF, Marchesini G. Insulin resistance in nonalcoholic fatty liver disease. Curr Pharm Des. 2010;16:1941–51.
Konner AC, Bruning JC. Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 2012;16:144–52.
Paschos P, Paletas K. Non alcoholic fatty liver disease and metabolic syndrome. Hippokratia. 2009;13:9–19.
Sanyal AJ. Mechanisms of disease: pathogenesis of nonalcoholic fatty liver disease. Nat Clin Pr Gastroenterol Hepatol. 2005;2:46–53.
Wang Y, Viscarra J, Kim SJ, Sul HS. Transcriptional regulation of hepatic lipogenesis. Nat Rev Mol Cell Biol. 2015;16:678–89.
Shao W, Espenshade PJ. Expanding roles for SREBP in metabolism. Cell Metab. 2012;16:414–9.
Wong RH, Sul HS. Insulin signaling in fatty acid and fat synthesis: a transcriptional perspective. Curr Opin Pharmacol. 2010;10:684–91.
Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Investig. 2002;109:1125–31.
Magana MM, Koo SH, Towle HC, Osborne TF. Different sterol regulatory element-binding protein-1 isoforms utilize distinct co-regulatory factors to activate the promoter for fatty acid synthase. J Biol Chem. 2000;275:4726–33.
Lopez JM, Bennett MK, Sanchez HB, Rosenfeld JM, Osborne TF. Sterol regulation of acetyl coenzyme A carboxylase: a mechanism for coordinate control of cellular lipid. Proc Natl Acad Sci U S A. 1996;93:1049–53.
Huang YY, Gusdon AM, Qu S. Nonalcoholic fatty liver disease: molecular pathways and therapeutic strategies. Lipids Health Dis. 2013;12:171.
Matsubara Y, Kraus JP, Yang-Feng TL, Francke U, Rosenberg LE, Tanaka K. Molecular cloning of cDNAs encoding rat and human medium-chain acyl-CoA dehydrogenase and assignment of the gene to human chromosome 1. Proc Natl Acad Sci U S A. 1986;83:6543–7.
Zhang Y, Ye J, Fan J. Regulation of malonyl-CoA-acyl carrier protein transacylase network in umbilical cord blood affected by intrauterine hyperglycemia. Oncotarget. 2017;8:75254–63.
Zhang L, Joshi AK, Smith S. Cloning, expression, characterization, and interaction of two components of a human mitochondrial fatty acid synthase. Malonyltransferase and acyl carrier protein. J Biol Chem. 2003;278:40067–74.
Mizokami A, Yasutake Y, Gao J, Matsuda M, Takahashi I, Takeuchi H, et al. Osteocalcin induces release of glucagon-like peptide-1 and thereby stimulates insulin secretion in mice. PLoS One. 2013;8:e57375.
Acknowledgements
This work was supported by the grant for a combined research and teaching program from the College of Life Sciences, University of Chinese Academy of Sciences (KJRH2015-006). We also thank the support of the CAMS Innovation Fund for Medical Sciences (CIFMS-2016-I2M-3-012) and the Drug Innovation Major Project (2018ZX09711001-003-005).
Author information
Authors and Affiliations
Contributions
XLZ and YNW conducted the pharmacological experiments; LYM and ZSL prepared uncarboxylated osteocalcin; XLZ, FY, and JHY designed the study, integrated the data, and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Zhang, Xl., Wang, Yn., Ma, Ly. et al. Uncarboxylated osteocalcin ameliorates hepatic glucose and lipid metabolism in KKAy mice via activating insulin signaling pathway. Acta Pharmacol Sin 41, 383–393 (2020). https://doi.org/10.1038/s41401-019-0311-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-019-0311-z
Keywords
This article is cited by
-
Mediating effect of osteocalcin underlying the link between insulin-like growth factor-I and gestational diabetes mellitus
BMC Pregnancy and Childbirth (2025)
-
Chemically synthesized osteocalcin alleviates NAFLD via the AMPK-FOXO1/BCL6-CD36 pathway
Journal of Translational Medicine (2024)
-
Empagliflozin ameliorates liver fibrosis in NASH rat model via targeting hepatic NF-κB/SOX9/OPN signaling and osteocalcin level
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Current knowledge of bone-derived factor osteocalcin: its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases
Journal of Molecular Medicine (2024)
-
Osteokines in Nonalcoholic Fatty Liver Disease
Current Obesity Reports (2024)