Abstract
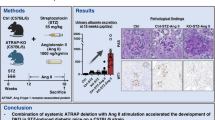
Some studies have shown that gut microbiota along with its metabolites is closely associated with diabetic mellitus (DM). In this study we explored the relationship between gut microbiota and kidney injuries of early diabetic nephropathy (DN) and its underlying mechanisms. Male SD rats were intraperitoneally injected with streptozotocin to induce DM. DM rats were orally administered compound broad-spectrum antibiotics for 8 weeks. After the rats were sacrificed, their blood, urine, feces, and renal tissues were harvested for analyses. We found that compared with the control rats, DM rats had abnormal intestinal microflora, increased plasma acetate levels, increased proteinuria, thickened glomerular basement membrane, and podocyte foot process effacement in the kidneys. Furthermore, the protein levels of angiotensin II, angiotensin-converting enzyme, and angiotensin II type 1 receptor in the kidneys of DM rats were significantly increased. Administration of broad-spectrum antibiotics in DM rats not only completely killed most intestinal microflora, but also significantly lowered the plasma acetate levels, inhibited intrarenal RAS activation, and attenuated kidney damage. Finally, we showed that plasma acetate levels were positively correlated with intrarenal angiotensin II protein expression (r = 0.969, P < 0.001). In conclusion, excessive acetate produced by disturbed gut microbiota might be involved in the kidney injuries of early DN through activating intrarenal RAS.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Cho NH, Shaw JE, Karuranga S, Huang Y, da Rocha Fernandes JD, Ohlrogge AW, et al. IDF Diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res Clin Pr. 2018;138:271–81.
Xu Y, Wang L, He J, Bi Y, Li M, Wang T, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–59.
Tamura J, Konno A, Hashimoto Y, Kon Y. Upregulation of renal renin–angiotensin system in mouse diabetic nephropathy. Jpn J Vet Res. 2005;53:13–26.
Kanasaki K, Taduri G, Koya D. Diabetic nephropathy: the role of inflammation in fibroblast activation and kidney fibrosis. Front Endocrinol. 2013;4:7.
Pichler R, Afkarian M, Dieter BP, Tuttle KR. Immunity and inflammation in diabetic kidney disease: translating mechanisms to biomarkers and treatment targets. Am J Physiol Ren Physiol. 2017;312:716–31.
Miranda-Diaz AG, Pazarin-Villasenor L, Yanowsky-Escatell FG, Andrade-Sierra J. Oxidative stress in diabetic nephropathy with early chronic kidney disease. J Diabetes Res. 2016;2016:7047238.
Urushihara M, Kagami S. Role of the intrarenal renin–angiotensin system in the progression of renal disease. Pediatr Nephrol. 2017;32:1471–79.
Schroeder BO, Backhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079–89.
Org E, Mehrabian M, Lusis AJ. Unraveling the environmental and genetic interactions in atherosclerosis: central role of the gut microbiota. Atherosclerosis. 2015;241:387–99.
Tilg H, Moschen AR. Microbiota and diabetes: an evolving relationship. Gut. 2014;63:1513–21.
Qin J, Li Y, Cai Z, Li S, Zhu J, Zhang F, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60.
Karlsson FH, Tremaroli V, Nookaew I, Bergstrom G, Behre CJ, Fagerberg B, et al. Gut metagenome in European women with normal, impaired and diabetic glucose control. Nature. 2013;498:99–103.
Louis P, Young P, Holtrop G, Flint HJ. Diversity of human colonic butyrate-producing bacteria revealed by analysis of the butyryl-CoA:acetate CoA-transferase gene. Environ Microbiol. 2010;12:304–14.
Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: links with metabolic and low-grade inflammation markers. Diabetes 2010;59:3049–57.
Vrieze A, Van Nood E, Holleman F, Salojarvi J, Kootte RS, Bartelsman JF, et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology. 2012;143:913–6.
Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085.
Murri M, Leiva I, Gomez-Zumaquero JM, Tinahones FJ, Cardona F, Soriguer F, et al. Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 2013;11:46.
Giongo A, Gano KA, Crabb DB, Mukherjee N, Novelo LL, Casella G, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J. 2011;5:82–91.
Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83.
Stilling RM, van de Wouw M, Clarke G, Stanton C, Dinan TG, Cryan JF. The neuropharmacology of butyrate: The bread and butter of the microbiota-gut-brain axis? Neurochem Int. 2016;99:110–32.
Vinolo MA, Rodrigues HG, Nachbar RT, Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3:858–76.
Kim MH, Kang SG, Park JH, Yanagisawa M, Kim CH. Short-chain fatty acids activate GPR41 and GPR43 on intestinal epithelial cells to promote inflammatory responses in mice. Gastroenterology. 2013;145:396–406.
Perry RJ, Peng L, Barry NA, Cline GW, Zhang D, Cardone RL, et al. Acetate mediates a microbiome-brain-beta-cell axis to promote metabolic syndrome. Nature. 2016;534:213–7.
Pluznick J, Protzko R, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA. 2013;110:4410–5.
Brown D, Sorscher EJ, Ausiello DA, Benos DJ. Immunocytochemical localization of Na+ channels in rat kidney medulla. Am J Physiol. 1989;256:366–9.
Kantorowicz L, Valego NK, Tang L, Figueroa JP, Chappell MC, Carey LC, et al. Plasma and renal renin concentrations in adult sheep after prenatal betamethasone exposure. Reprod Sci. 2008;15:831–8.
Hostetter TH. Progression of renal disease and renal hypertrophy. Annu Rev Physiol. 1995;57:263–78.
Mogensen CE, Christensen CK, Vittinghus E. The stages in diabetic renal disease. With emphasis on the stage of incipient diabetic nephropathy. Diabetes. 1983;32:64–78.
Kumar R, Thomas CM, Yong QC, Chen W, Baker KM. The intracrine renin–angiotensin system. Clin Sci. 2012;123:273–84.
Hunyady L, Catt KJ. Pleiotropic AT1 receptor signaling pathways mediating physiological and pathogenic actions of angiotensin II. Mol Endocrinol. 2006;20:953–70.
Wysocki J, Ye M, Khattab AM, Fogo A, Martin A, David NV, et al. Angiotensin-converting enzyme 2 amplification limited to the circulation does not protect mice from development of diabetic nephropathy. Kidney Int. 2017;91:1336–46.
Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev. 1996;76:425–536.
Carey RM, Siragy HM. The intrarenal renin–angiotensin system and diabetic nephropathy. Trends Endocrinol Metab. 2003;14:274–81.
Jia L, Li D, Feng N, Shamoon M, Sun Z, Ding L, et al. Anti-diabetic effects of clostridium butyricum CGMCC0313.1 through promoting the growth of gut butyrate-producing bacteria in type 2 diabetic mice. Sci Rep. 2017;7:7046.
Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–96.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (grant 81970629), the Jiangsu Province Social Development Project (BE2018744), the Project for Jiangsu Provincial Medical Talent (ZDRCA2016077), the Jiangsu Province Six Talent Peaks Project (2015-WSN-002), the Fundamental Research Funds for the Central Universities (KYCX18-0182, KYCX17-0169, KYZZ15-0061), the Jiangsu Province Ordinary University Graduate Research Innovation Project (SJZZ16-004), and the Joint Project of Southeast University and Pharmaceutical University of China (2242019K3DZ03).
Author information
Authors and Affiliations
Contributions
CCL performed the research, analyzed the data, and wrote the paper; KLM designed and reviewed the paper; ZBH, RW, ZHH, JL, PPC, JXZ, XQL, BYY, and SJH assisted in the research; XZR and BCL analyzed and interpreted the data. All authors read and approved the final paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Lu, Cc., Hu, Zb., Wang, R. et al. Gut microbiota dysbiosis-induced activation of the intrarenal renin–angiotensin system is involved in kidney injuries in rat diabetic nephropathy. Acta Pharmacol Sin 41, 1111–1118 (2020). https://doi.org/10.1038/s41401-019-0326-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-019-0326-5
Keywords
This article is cited by
-
Causal effect of gut microbiota and diabetic nephropathy: a Mendelian randomization study
Diabetology & Metabolic Syndrome (2024)
-
Upregulation of UHRF1 Promotes PINK1-mediated Mitophagy to Alleviates Ferroptosis in Diabetic Nephropathy
Inflammation (2024)
-
Gut microbiota in relationship to diabetes mellitus and its late complications with a focus on diabetic foot syndrome: A review
Folia Microbiologica (2024)
-
The potential mechanism of gut microbiota-microbial metabolites-mitochondrial axis in progression of diabetic kidney disease
Molecular Medicine (2023)
-
Microbiome modulation in inflammatory diseases: Progress to microbiome genetic engineering
Cancer Cell International (2023)