Abstract
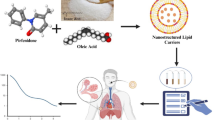
Sepsis is a dysregulated immune response to infection and potentially leads to life-threatening organ dysfunction, which is often seen in serious Covid-19 patients. Disulfiram (DSF), an old drug that has been used to treat alcohol addiction for decades, has recently been identified as a potent inhibitor of the gasdermin D (GSDMD)-induced pore formation that causes pyroptosis and inflammatory cytokine release. Therefore, DSF represents a promising therapeutic for the treatment of inflammatory disorders. Lactoferrin (LF) is a multifunctional glycoprotein with potent antibacterial and anti-inflammatory activities that acts by neutralizing circulating endotoxins and activating cellular responses. In addition, LF has been well exploited as a drug nanocarrier and targeting ligands. In this study, we developed a DSF-LF nanoparticulate system (DSF-LF NP) for combining the immunosuppressive activities of both DSF and LF. DSF-LF NPs could effectively block pyroptosis and inflammatory cytokine release from macrophages. Treatment with DSF-LF NPs showed remarkable therapeutic effects on lipopolysaccharide (LPS)-induced sepsis. In addition, this therapeutic strategy was also applied to treat ulcerative colitis (UC), and substantial treatment efficacy was achieved in a murine colitis model. The underlying mode of action of these DSF-LF-NPs may contribute to efficiently suppressing macrophage-mediated inflammatory responses and ameliorating the complications caused by sepsis and UC. As macrophage pyroptosis plays a pivotal role in inflammation, this safe and effective biomimetic nanomedicine may offer a versatile therapeutic strategy for treating various inflammatory diseases by repurposing DSF.

Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315:801–10.
Rudd KE, Johnson SC, Agesa KM, Shackelford KA, Tsoi D, Kievlan DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. 2020;395:200–11.
Xie J, Wang H, Kang Y, Zhou L, Liu Z, Qin B, et al. The epidemiology of sepsis in Chinese ICUs: a national cross-sectional survey. Crit Care Med. 2020;48:e209–e18.
Lin GL, McGinley JP, Drysdale SB, Pollard AJ. Epidemiology and immune pathogenesis of viral sepsis. Front Immunol. 2018;9:2147.
Li H, Liu L, Zhang D, Xu J, Dai H, Tang N, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395:1517–20.
Ponsford MJ, Gkatzionis A, Walker VM, Grant AJ, Wootton RE, Moore LSP, et al. Cardiometabolic traits, sepsis, and severe COVID-19: a mendelian randomization investigation. Circulation. 2020;142:1791–3.
Remy KE, Brakenridge SC, Francois B, Daix T, Deutschman CS, Monneret G, et al. Immunotherapies for COVID-19: lessons learned from sepsis. Lancet Respir Med. 2020;8:946–9.
Hotchkiss RS, Moldawer LL, Opal SM, Reinhart K, Turnbull IR, Vincent JL. Sepsis and septic shock. Nat Rev Dis Prim. 2016;2:16045.
Lelubre C, Vincent JL. Mechanisms and treatment of organ failure in sepsis. Nat Rev Nephrol. 2018;14:417–27.
Ma KC, Schenck EJ, Pabon MA, Choi AMK. The role of danger signals in the pathogenesis and perpetuation of critical illness. Am J Respir Crit Care Med. 2018;197:300–9.
Andersson U, Tracey KJ. HMGB1 is a therapeutic target for sterile inflammation and infection. Annu Rev Immunol. 2011;29:139–62.
Deutschman CS, Tracey KJ. Sepsis: current dogma and new perspectives. Immunity. 2014;40:463–75.
Sborgi L, Rühl S, Mulvihill E, Pipercevic J, Heilig R, Stahlberg H, et al. GSDMD membrane pore formation constitutes the mechanism of pyroptotic cell death. EMBO J. 2016;35:1766–78.
Liu X, Zhang Z, Ruan J, Pan Y, Magupalli VG, Wu H, et al. Inflammasome-activated gasdermin D causes pyroptosis by forming membrane pores. Nature. 2016;535:153–8.
Lamkanfi M, Dixit VM. Mechanisms and functions of inflammasomes. Cell. 2014;157:1013–22.
He WT, Wan H, Hu L, Chen P, Wang X, Huang Z, et al. Gasdermin D is an executor of pyroptosis and required for interleukin-1beta secretion. Cell Res. 2015;25:1285–98.
Hu JJ, Liu X, Xia S, Zhang Z, Zhang Y, Zhao J, et al. FDA-approved disulfiram inhibits pyroptosis by blocking gasdermin D pore formation. Nat Immunol. 2020;21:736–45.
Deng W, Yang Z, Yue H, Ou Y, Hu W, Sun P. Disulfiram suppresses NLRP3 inflammasome activation to treat peritoneal and gouty inflammation. Free Radic Biol Med. 2020;152:8–17.
Johansson B. A review of the pharmacokinetics and pharmacodynamics of disulfiram and its metabolites. Acta Psychiatr Scand Suppl. 1992;86:15–26.
Duan X, Xiao J, Yin Q, Zhang Z, Yu H, Mao S, et al. Smart pH-sensitive and temporal-controlled polymeric micelles for effective combination therapy of doxorubicin and disulfiram. ACS Nano. 2013;7:5858–69.
Deng N, Li M, Shen D, He Q, Sun W, Liu M, et al. LRP1 receptor-mediated immunosuppression of alpha-MMC on monocytes. Int Immunopharmacol. 2019;70:80–7.
Manzoni P, Dall’Agnola A, Tomé D, Kaufman DA, Tavella E, Pieretto M, et al. Role of lactoferrin in neonates and infants: an update. Am J Perinatol. 2018;35:561–5.
Elzoghby AO, Abdelmoneem MA, Hassanin IA, Abd Elwakil MM, Elnaggar MA, Mokhtar S, et al. Lactoferrin, a multi-functional glycoprotein: active therapeutic, drug nanocarrier & targeting ligand. Biomaterials. 2020;263:120355.
Kobayashi T, Siegmund B, Le Berre C, Wei SC, Ferrante M, Shen B, et al. Ulcerative colitis. Nat Rev Dis Prim. 2020;6:74.
Ng SC, Shi HY, Hamidi N, Underwood FE, Tang W, Benchimol EI, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2017;390:2769–78.
Olén O, Erichsen R, Sachs MC, Pedersen L, Halfvarson J, Askling J, et al. Colorectal cancer in ulcerative colitis: a Scandinavian population-based cohort study. Lancet. 2020;395:123–31.
Bopanna S, Ananthakrishnan AN, Kedia S, Yajnik V, Ahuja V. Risk of colorectal cancer in Asian patients with ulcerative colitis: a systematic review and meta-analysis. Lancet. Gastroenterol Hepatol. 2017;2:269–76.
Cohen J, Vincent JL, Adhikari NK, Machado FR, Angus DC, Calandra T, et al. Sepsis: a roadmap for future research. Lancet Infect Dis. 2015;15:581–614.
Gotts JE, Matthay MA. Sepsis: pathophysiology and clinical management. BMJ. 2016;353:i1585.
Steinhagen F, Schmidt SV, Schewe JC, Peukert K, Klinman DM, Bode C. Immunotherapy in sepsis - brake or accelerate? Pharmacol Ther. 2020;208:107476.
Fink MP. Animal models of sepsis and its complications. Kidney Int. 2008;74:991–3.
Drago-Serrano ME, de la Garza-Amaya M, Luna JS, Campos-Rodriguez R. Lactoferrin-lipopolysaccharide (LPS) binding as key to antibacterial and antiendotoxic effects. Int Immunopharmacol. 2012;12:1–9.
Kruzel ML, Harari Y, Mailman D, Actor JK, Zimecki M. Differential effects of prophylactic, concurrent and therapeutic lactoferrin treatment on LPS-induced inflammatory responses in mice. Clin Exp Immunol. 2002;130:25–31.
Doursout MF, Horton H, Hoang L, Liang Y, Hwang SA, Boyd S, et al. Lactoferrin moderates LPS-induced hypotensive response and gut injury in rats. Int Immunopharmacol. 2013;15:227–31.
Kruzel ML, Zimecki M, Actor JK. Lactoferrin in a context of inflammation-induced pathology. Front Immunol. 2017;8:1438.
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25.
Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF. Ulcerative colitis. Lancet. 2017;389:1756–70.
Peyrin-Biroulet L, Lémann M. Review article: remission rates achievable by current therapies for inflammatory bowel disease. Aliment Pharmacol Ther. 2011;33:870–9.
Na YR, Stakenborg M, Seok SH, Matteoli G. Macrophages in intestinal inflammation and resolution: a potential therapeutic target in IBD. Nat Rev Gastroenterol Hepatol. 2019;16:531–43.
Mowat AM. To respond or not to respond - a personal perspective of intestinal tolerance. Nat Rev Immunol. 2018;18:405–15.
Zhao Y, Yang Y, Zhang J, Wang R, Cheng B, Kalambhe D, et al. Lactoferrin-mediated macrophage targeting delivery and patchouli alcohol-based therapeutic strategy for inflammatory bowel diseases. Acta Pharm Sin B. 2020;10:1966–76.
Davis EM, Zhang D, Glover SC, Stappenbeck TS, Liu JJ. Su1832 – inhibition of intestinal epithelial cell pyroptosis and associated mucosal barrier defects is a potential therapeutic mechanism of action for mesalamine in Ibd. Gastroenterology. 2019;156:S–627.
Ren TH, Lv MM, An XM, Leung WK, Seto WK. Activation of adenosine A3 receptor inhibits inflammatory cytokine production in colonic mucosa of patients with ulcerative colitis by down-regulating the nuclear factor-kappa B signaling. J Dig Dis. 2020;21:38–45.
Wu X, Pan S, Luo W, Shen Z, Meng X, Xiao M, et al. Roseburia intestinalis‑derived flagellin ameliorates colitis by targeting miR‑223‑3p‑mediated activation of NLRP3 inflammasome and pyroptosis. Mol Med Rep. 2020;22:2695–704.
Acknowledgements
We are thankful for the support from National Key Research and Development Program of China (2021YFE0103100, China), NFSC (81925035 and 81521005), and National Special Project for Significant New Drugs Development (2018ZX09711002-010-002), and Shanghai SciTech Innovation Initiative (19431903100, 18430740800) and Shanghai Collaborative Innovation Group of Early Diagnosis and Precise Treatment of Hemangiomas and Vascular Malformations (SSMU-ZDCX20180701) for the support. We thank the Molecular Imaging Center and TEM Facility at SIMM and the National Center for Protein Science Shanghai, CAS, for the technical support at flow cytometry and MALDI-TOF MASS Facility.
Author information
Authors and Affiliations
Contributions
ATO: Investigation, Data curation, Formal analysis, Writing original draft. JXZ and YFF: Investigation, Visualization, Methodology. RW and XPT: Investigation, Validation. PFZ, YGZ, MZ: Methodology, Validation. YZH: Formal analysis, Project administration, Supervision, Writing—review & editing.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Ou, At., Zhang, Jx., Fang, Yf. et al. Disulfiram-loaded lactoferrin nanoparticles for treating inflammatory diseases. Acta Pharmacol Sin 42, 1913–1920 (2021). https://doi.org/10.1038/s41401-021-00770-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-021-00770-w
Keywords
This article is cited by
-
AuCePt porous hollow cascade nanozymes targeted delivery of disulfiram for alleviating hepatic insulin resistance
Journal of Nanobiotechnology (2024)
-
Disulfiram-loaded nanovesicles hydrogel promotes healing of diabetic wound
Journal of Translational Medicine (2024)
-
The gasdermin family: emerging therapeutic targets in diseases
Signal Transduction and Targeted Therapy (2024)
-
The gasdermin protein family: emerging roles in gastrointestinal health and disease
Nature Reviews Gastroenterology & Hepatology (2023)
-
The immunomodulatory function and antitumor effect of disulfiram: paving the way for novel cancer therapeutics
Discover Oncology (2023)