Abstract
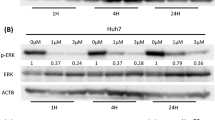
Recent studies show that liver X receptor (LXR) agonists exert significant antitumor effects in a variety of tumor cell lines including hepatocellular carcinoma (HCC). But the molecular mechanisms underlying LXR antitumor activity are not fully understood. In this study we investigated the effect of LXR agonist T0901317 (T317) on HCC development and its relationship with RalA binding protein 1 (RALBP1)-associated EPS ___domain containing 2 (REPS2)/epidermal growth factor receptor (EGFR) signaling axis. We showed that T317 (0.1−0.5 μM) dose-dependently increased REPS2 expression in normal hepatocytes (BNLCL.2 and LO2) and HCC cells (HepG2 and Huh-7). Using promoter activity assay and chromatin immunoprecipitation (CHIP) assay we demonstrated that T317 enhanced REPS2 expression at the transcriptional level via promoting the binding of LXR protein to the LXR-response element (LXRE) in the REPS2 promoter region. We showed that the inhibitory effect of T317 on the proliferation and migration of HCC cells was closely related to REPS2. Moreover, we revealed that T317 (400 nM) increased expression of REPS2 in HepG2 cells, thus inhibiting epidermal growth factor (EGF)-mediated endocytosis of EGFR as well as the downstream activation of AKT/NF-κB, p38MAPK, and ERK1/2 signaling pathways. Clinical data analysis revealed that REPS2 expression levels were inversely correlated with the development of HCC and reduced REPS2 expression associated with poor prognosis, suggesting that REPS2 might be involved in the development of HCC. In conclusion, this study provides new insights into the potential mechanisms of LXR agonist-inhibited HCC.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
Data availability
All data shown are available in the article and the supporting information.
References
Apfel R, Benbrook D, Lernhardt E, Ortiz MA, Salbert G, Pfahl M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol Cell Biol. 1994;14:7025–35.
Willy PJ, Umesono K, Ong ES, Evans RM, Heyman RA, Mangelsdorf DJ. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Gene Dev. 1995;9:1033–45.
Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem. 1997;272:3137–40.
Calkin AC, Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–24.
Attie AD. ABCA1: at the nexus of cholesterol, HDL and atherosclerosis. Trends Biochem Sci. 2007;32:172–9.
Tarling EJ. Expanding roles of ABCG1 and sterol transport. Curr Opin Lipido. 2013;24:138–46.
Andrade RJ, Agúndez JAG, Lucena MI, Martínez C, Cueto R, García-Martín E. Pharmacogenomics in drug induced liver injury. Curr Drug Metab. 2009;10:956–70.
Rudkowska I, Jones PJ. Polymorphisms in ABCG5/G8 transporters linked to hypercholesterolemia and gallstone disease. Nutr Rev. 2008;66:343–8.
Joseph SB, Castrillo A, Laffitte BA, Mangelsdorf DJ, Tontonoz P. Reciprocal regulation of inflammation and lipid metabolism by liver X receptors. Nat Med. 2003;9:213–9.
Joseph SB, Bradley MN, Castrillo A, Bruhn KW, Mak PA, Pei L, et al. LXR-dependent gene expression is important for macrophage survival and the innate immune response. Cell. 2004;119:299–309.
El Roz A, Bard JM, Huvelin JM, Nazih H. LXR agonists and ABCG1-dependent cholesterol efflux in MCF-7 breast cancer cells: relation to proliferation and apoptosis. Anticancer Res. 2012;32:3007–13.
Sasso GL, Bovenga F, Murzilli S, Salvatore L, Tullio GD, Martelli N, et al. Liver X receptors inhibit proliferation of human colorectal cancer cells and growth of intestinal tumors in mice. Gastroenterology. 2013;144:1497–507.
Chuu CP, Lin HP. Antiproliferative effect of LXR agonists T0901317 and 22(R)-hydroxycholesterol on multiple human cancer cell lines. Anticancer Res. 2010;30:3643–8.
He J, Yang T, He W, Jiang S, Zhong D, Xu Z, et al. Liver X receptor inhibits the growth of hepatocellular carcinoma cells via regulating HULC/miR-134-5p/FOXM1 axis. Cell Signal. 2020;74:109720.
Morén A, Bellomo C, Tsubakihara Y, Kardassis D, Mikulits W, Heldin CH, et al. LXRα limits TGFβ-dependent hepatocellular carcinoma associated fibroblast differentiation. Oncogenesis. 2019;8:36.
Ikeda M, Ishida O, Hinoi T, Kishida S, Kikuchi A. Identification and characterization of a novel protein interacting with ral-binding protein 1, a putative effector protein of ral. J Biol Chem. 1998;273:814–21.
Nakashima S, Morinaka K, Koyama S, Ikeda M, Kishida M, Okawa K, et al. Small G protein Ral and its downstream molecules regulate endocytosis of EGF and insulin receptors. EMBO J. 1999;18:3629–42.
Morinaka K, Koyama S, Nakashima S, Hinoi T, Okawa K, Iwamatsu A, et al. Epsin binds to the EH ___domain of POB1 and regulates receptor-mediated endocytosis. Oncogene. 1999;18:5915–22.
Oosterhoff JK, Penninkhof F, Brinkmann AO, Anton Grootegoed J, Blok LJ. REPS2/POB1 is downregulated during human prostate cancer progression and inhibits growth factor signalling in prostate cancer cells. Oncogene. 2003;22:2920–5.
Penninkhof F, Grootegoed JA, Blok LJ. Identification of REPS2 as a putative modulator of NF-κB activity in prostate cancer cells. Oncogene. 2004;23:5607–15.
Doolan P, Clynes M, Kennedy S, Mehta JP, Germano S, Ehrhardt C, et al. TMEM25, REPS2 and Meis 1: favourable prognostic and predictive biomarkers for breast cancer. Tumor Biol. 2009;30:200–9.
Li F, Ji JP, Xu Y, Liu RL. Identification a novel set of 6 differential expressed genes in prostate cancer that can potentially predict biochemical recurrence after curative surgery. Clin Transl Oncol. 2019;21:1067–75.
Zhang H, Duan CJ, Zhang H, Cheng YD, Zhang CF. Expression and clinical significance of REPS2 in human esophageal squamous cell carcinoma. Asian Pac J Cancer Prev. 2013;14:2851–7.
Limaye PB, Bowen WC, Orr AV, Luo J, Tseng GC, Michalopoulos GK. Mechanisms of hepatocyte growth factor-mediated and epidermal growth factor-mediated signaling in transdifferentiation of rat hepatocytes to biliary epithelium. Hepatology. 2010;47:1702–13.
Shiraga M, Komatsu N, Teshigawara K, Okada A, Takeuchi S, Fukamachi H, et al. Epidermal growth factor stimulates proliferation of mouse uterine epithelial cells in primary culture. Zool Sci. 2000;17:661–6.
Berasain C, Perugorria MJ, Latasa MU, Castillo J, Goni S, Santamaria M, et al. The epidermal growth factor receptor: a link between inflammation and liver cancer. Exp Biol Med. 2009;234:713–25.
Berasain C, Avila MA. The EGFR signalling system in the liver: from hepatoprotection to hepatocarcinogenesis. J Gastroenterol. 2014;49:9–23.
Xiong T, Li Z, Huang X, Lu K, Xie W, Zhou Z, et al. TO901317 inhibits the development of hepatocellular carcinoma by LXRα/Glut1 decreasing the glycometabolism. Am J Physiol Gastrointest Liver Physiol. 2019;316:G598–607.
Liu Y, Wei Z, Zhang Y, Ma X, Chen Y, Yu M, et al. Activation of liver X receptor plays a central role in antiviral actions of 25-hydroxycholesterol. J Lipid Res. 2018;59:2287–96.
Percie du Sert N, Hurst V, Ahluwalia A, Alam S, Avey MT, Baker M, et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br J Pharmacol. 2020;177:3617–24.
Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG, Group NCRRGW. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–9.
Chen Y, Duan Y, Kang Y, Yang X, Jiang M, Zhang L, et al. Activation of liver X receptor induces macrophage interleukin-5 expression. J Biol Chem. 2012;287:43340–50.
Pinskiy V, Tolpygo AS, Jones J, Weber K, Franciotti N, Mitra PP. A low-cost technique to cryo-protect and freeze rodent brains, precisely aligned to stereotaxic coordinates for whole-brain cryosectioning. J Neurosci Methods. 2013;218:206–13.
Yang G, Huang L, Jia H, Aikemu B, Zhang S, Shao Y, et al. NDRG1 enhances the sensitivity of cetuximab by modulating EGFR trafficking in colorectal cancer. Oncogene. 2021;40:5993–6006.
Badway JA, Baleja JD. REPS2: A cellular signaling and molecular trafficking nexus. Int J Biochem Cell Biol. 2011;43:1660–3.
Lin C-Y, Vedin L-L, Steffensen KR. The emerging roles of liver X receptors and their ligands in cancer. Expert Opin Ther Targets. 2016;20:61–71.
Bartha Á, Győrffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. Int J Mol Sci. 2021;22:2622.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Carbó JM, León TE, Font-Díaz J, De la Rosa JV, Castrillo A, Picard FR, et al. Pharmacologic activation of LXR alters the expression profile of tumor-associated macrophages and the abundance of regulatory T cells in the tumor microenvironment. Cancer Res. 2021;81:968–85.
Shiragannavar VD, Gowda NGS, Santhekadur PK. Discovery of eukaryotic cellular receptor for Withaferin A, a multifaceted drug from Withania somnifera plant. Med Drug Discov. 2022;14:100127.
Shiragannavar VD, Gowda NGS, Kumar DP, Mirshahi F, Santhekadur PK. Withaferin a acts as a novel regulator of liver x receptor-alpha in HCC. Front Oncol. 2020;10:628506.
Tomassi L, Costantini A, Corallino S, Santonico E, Carducci M, Cesareni G, et al. The central proline rich region of POB1/REPS2 plays a regulatory role in epidermal growth factor receptor endocytosis by binding to 14-3-3 and SH3 ___domain-containing proteins. BMC Biochem. 2008;9:21.
Singhal SS, Yadav S, Drake K, Singhal J, Awasthi S. Hsf-1 and POB1 induce drug sensitivity and apoptosis by inhibiting Ralbp1. J Biol Chem. 2008;283:19714–29.
Schlessinger J. Ligand-induced, receptor-mediated dimerization and activation of EGF receptor. Cell. 2002;110:669–72.
Rnja C, Fwa C, Np A, Tpjgb C, Cww CD, Awba C. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53.
Shao W, Zhu W, Lin J, Luo M, Lin Z, Lu L, et al. Liver X receptor agonism sensitizes a subset of hepatocellular carcinoma to sorafenib by dual-inhibiting MET and EGFR. Neoplasia. 2020;22:1–9.
Schultz JR, Tu H, Luk A, Repa JJ, Medina JC, Li L, et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000;14:2831–8.
Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–74.
Delire B, Stärkel P. The Ras/MAPK pathway and hepatocarcinoma: pathogenesis and therapeutic implications. Eur J Clin Invest. 2015;45:609–23.
Freed DM, Bessman NJ, Kiyatkin A, Salazar-Cavazos E, Byrne PO, Moore JO, et al. EGFR ligands differentially stabilize receptor dimers to specify signaling kinetics. Cell. 2017;171:683–95.e18.
Acknowledgements
This work was funded by the National Natural Science Foundation of China grants 31770863 to YLC, 81973316 to JHH, and 81803517 to XXY.
Author information
Authors and Affiliations
Contributions
Conceptualization: YLC and JHH; methodology and investigation: XYH, MMZ, JZ, CYW, XKZ, SZ, XXY, and YJD; software: XYH and BTZ; clinical sample collection: DCZ; writing—original draft preparation: XYH and MMZ; writing—review and editing: YLC; supervision: YLC; funding acquisition: YLC, XXY, and JHH. All authors have read and agreed to the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, Xy., Zhu, Mm., Zheng, J. et al. Liver X receptor agonists exert antitumor effects against hepatocellular carcinoma via inducing REPS2 expression. Acta Pharmacol Sin 44, 635–646 (2023). https://doi.org/10.1038/s41401-022-00961-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41401-022-00961-z
Keywords
This article is cited by
-
REPS2 attenuates cancer stemness through inhibiting Wnt signaling by autophagy mediated degradation of β-catenin
Oncogene (2025)
-
Multi-ancestry genome-wide association study of kidney cancer identifies 63 susceptibility regions
Nature Genetics (2024)
-
Dysregulated cholesterol regulatory genes in hepatocellular carcinoma
European Journal of Medical Research (2023)