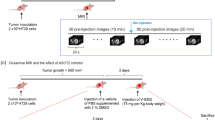
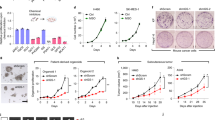
Abstract
Head and neck squamous cell carcinoma (HNSCC) cells exhibit a high dependency on glutamine metabolism, making it an attractive target. Despite the well-established link between glutamine reliance and tumor progression, the specific role of glutamine transporters in HNSCC remains poorly understood. The alanine-serine-cysteine transporter 2 (ASCT2), a key glutamine transporter, is overexpressed in HNSCC, and its silencing has been shown to reduce intracellular glutamine and glutathione levels, inhibiting tumor growth. These facts suggest that targeting ASCT2-mediated glutamine uptake could offer a promising therapeutic strategy for HNSCC. But no clinically approved drugs directly target ASCT2, and challenges such as the limited stability of antisense oligonucleotides persist. In this study we evaluated the correlation between ASCT2-mediate glutamine metabolism and its impact on HNSCC patients. We established a virtual screening method followed by cytotoxic assays to identify small molecules that specifically target ASCT2. Among the top 15 candidates, we identified yuanhuacine (YC) as the most potent antitumor compound with IC50 values of 1.43, 6.62, and 6.46 μM against HN6, CAL33, and SCC7 cells, respectively. We demonstrated that YC (0.3–5 μM) dose-dependently induced ASCT2 degradation by recruiting the E3 ubiquitin ligase RNF5, inhibiting glutamine uptake in HN6 cells. This disruption led to mitochondrial dysfunction and enhanced the therapeutic efficacy of YC. Our results highlight YC as a promising regulator of ASCT2-mediated glutamine metabolism in HNSCC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Khera NRA, Abdulkader M Alkurdi K, Liu Z, Ma H, Waseem A, Teh MT. Identification of multidrug chemoresistant genes in head and neck squamous cell carcinoma cells. Mol Cancer. 2023;22:146.
Chow LQM. Head and neck cancer. N Engl J Med. 2020;382:60–72.
Ludwig R, Pouymayou B, Balermpas P, Unkelbach J. A hidden Markov model for lymphatic tumor progression in the head and neck. Sci Rep. 2021;11:12261.
Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discov. 2022;12:31–46.
Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–34.
Zhu L, Ploessl K, Zhou R, Mankoff D, Kung HF. Metabolic imaging of glutamine in cancer. J Nucl Med. 2017;58:533–7.
Sagardoy T, Fernandez P, Ghafouri A, Digue L, Haaser T, de Clermont-Galleran H. et al. Accuracy of (18) FDG PET-CT for treatment evaluation 3 months after completion of chemoradio therapy for head and neck squamous cell carcinoma: 2-year minimum follow-up. Head Neck. 2016;38:E1271–6.
Zhang Z, Liu R, Shuai Y, Huang Y, Jin R, Wang X, et al. ASCT2 (SLC1A5)-dependent glutamine uptake is involved in the progression of head and neck squamous cell carcinoma. Br J Cancer. 2020;122:82–93.
Luo Y, Li W, Ling Z, Hu Q, Fan Z, Cheng B, et al. ASCT2 overexpression is associated with poor survival of OSCC patients and ASCT2 knockdown inhibited growth of glutamine-addicted OSCC cells. Cancer Med. 2020;9:3489–99.
Beddok A, Orlhac F, Calugaru V, Champion L, Ala Eddine C, Nioche C, et al. [18F]-FDG PET and MRI radiomic signatures to predict the risk and the ___location of tumor recurrence after re-irradiation in head and neck cancer. Eur J Nucl Med Mol I. 2023;50:559–71.
Yang L, Venneti S, Nagrath D. Glutaminolysis: a hallmark of cancer metabolism. Annu Rev Biomed Eng. 2017;19:163–94.
Luo JT, Wang YF, Wang Y, Wang CL, Liu RY, Zhang Z. CircMAT2B facilitates the progression of head and neck squamous cell carcinoma via sponging miR-491-5p to trigger ASCT2-mediated glutaminolysis. Mol Cell Biochem. 2023;478:1067–81.
Yang J, Guo Y, Seo W, Zhang R, Lu C, Wang Y, et al. Targeting cellular metabolism to reduce head and neck cancer growth. Sci Rep. 2019;9:4995.
Ai C, Sun X, Xiao S, Guo L, Shang M, Shi D, et al. CAFs targeted ultrasound-responsive nanodroplets loaded V9302 and GLULsiRNA to inhibit melanoma growth via glutamine metabolic reprogramming and tumor microenvironment remodeling. J Nanobiotechnol. 2023;21:214.
Wu S, Fukumoto T, Lin J, Nacarelli T, Wang Y, Ong D, et al. Targeting glutamine dependence through GLS1 inhibition suppresses ARID1A-inactivated clear cell ovarian carcinoma. Nat Cancer. 2021;2:189–200.
Khoury ES, Sharma A, Ramireddy RR, Thomas AG, Alt J, Fowler A, et al. Dendrimer-conjugated glutaminase inhibitor selectively targets microglial glutaminase in a mouse model of Rett syndrome. Theranostics. 2020;10:5736–48.
Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: understanding the heterogeneity. Trends Cancer. 2017;3:169–80.
Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52:1496–516.
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–33.
Yang J, Chen F, Lang L, Yang F, Fu Z, Martinez J, et al. Therapeutic targeting of the GLS1-c-Myc positive feedback loop suppresses glutaminolysis and inhibits progression of head and neck cancer. Cancer Res. 2024;84:3223–34.
El Ansari R, McIntyre A, Craze ML, Ellis IO, Rakha EA, Green AR. Altered glutamine metabolism in breast cancer; subtype dependencies and alternative adaptations. Histopathology. 2018;72:183–90.
Mukha A, Kahya U, Dubrovska A. Targeting glutamine metabolism and autophagy: the combination for prostate cancer radiosensitization. Autophagy. 2021;17:3879–81.
Zhang J, Pavlova NN, Thompson CB. Cancer cell metabolism: the essential role of the nonessential amino acid, glutamine. EMBO J. 2017;36:1302–15.
Pacifico F, Leonardi A, Crescenzi E. Glutamine metabolism in cancer stem cells: a complex liaison in the tumor microenvironment. Int J Mol Sci. 2023;24:2337.
Cha YJ, Kim ES, Koo JS. Amino acid transporters and glutamine metabolism in breast cancer. Int J Mol Sci. 2018;19:907.
Gray KA, Seal RL, Tweedie S, Wright MW, Bruford EA. A review of the new HGNC gene family resource. Hum Genomics. 2016;10:6.
Yoo HC, Park SJ, Nam M, Kang J, Kim K, Yeo JH, et al. A variant of SLC1A5 is a mitochondrial glutamine transporter for metabolic reprogramming in cancer cells. Cell Metab. 2020;31:267–83.e12.
Li L, Nie L, Jordan A, Cai Q, Liu Y, Li Y, et al. Targeting glutaminase is therapeutically effective in ibrutinib-resistant mantle cell lymphoma. Haematologica. 2022;108:1616–27.
Ni F, Yu W-M, Li Z, Graham DK, Jin L, Kang S, et al. Critical role of ASCT2-mediated amino acid metabolism in promoting leukaemia development and progression. Nat Metab. 2019;1:390–403.
Teixeira E, Silva C, Martel F. The role of the glutamine transporter ASCT2 in antineoplastic therapy. Cancer Chemoth Pharmacol. 2021;87:447–64.
Hara Y, Minami Y, Yoshimoto S, Hayashi N, Yamasaki A, Ueda S, et al. Anti-tumor effects of an antagonistic mAb against the ASCT2 amino acid transporter on KRAS-mutated human colorectal cancer cells. Cancer Med. 2020;9:302–12.
Suzuki M, Toki H, Furuya A, Ando H. Establishment of monoclonal antibodies against cell surface domains of ASCT2/SLC1A5 and their inhibition of glutamine-dependent tumor cell growth. Biochem Biophys Res Commun. 2017;482:651–7.
Oppedisano F, Catto M, Koutentis PA, Nicolotti O, Pochini L, Koyioni M, et al. Inactivation of the glutamine/amino acid transporter ASCT2 by 1,2,3-dithiazoles: proteoliposomes as a tool to gain insights in the molecular mechanism of action and of antitumor activity. Toxicol Appl Pharmacol. 2012;265:93–102.
Taurino G, Dander E, Chiu M, Pozzi G, Maccari C, Starace R, et al. Asparagine transport through SLC1A5/ASCT2 and SLC38A5/SNAT5 is essential for BCP-ALL cell survival and a potential therapeutic target. Br J Haematol. 2024;205:175–88.
Bröer A, Fairweather S, Bröer S. Disruption of amino acid homeostasis by novel ASCT2 inhibitors involves multiple targets. Front Pharmacol. 2018;9:785.
Cormerais Y, Massard PA, Vucetic M, Giuliano S, Tambutté E, Durivault J, et al. The glutamine transporter ASCT2 (SLC1A5) promotes tumor growth independently of the amino acid transporter LAT1 (SLC7A5). J Biol Chem. 2018;293:2877–87.
Pan T, Huang B, Zhang W, Gabos S, Huang DY, Devendran V. Cytotoxicity assessment based on the AUC50 using multi-concentration time-dependent cellular response curves. Anal Chim Acta. 2013;764:44–52.
Jiang L, Yin X, Chen Y-H, Chen Y, Jiang W, Zheng H, et al. Proteomic analysis reveals ginsenoside Rb1 attenuates myocardial ischemia/reperfusion injury through inhibiting ROS production from mitochondrial complex I. Theranostics. 2021;11:1703–20.
Zhang C, Li K, Zhu H, Cheng M, Chen S, Ling R, et al. ITGB6 modulates resistance to anti-CD276 therapy in head and neck cancer by promoting PF4+ macrophage infiltration. Nat Commun. 2024;15:7077.
Cai H, Liang J, Jiang Y, Wang Z, Li H, Wang W, et al. KLF7 regulates super-enhancer-driven IGF2BP2 overexpression to promote the progression of head and neck squamous cell carcinoma. J Exp Clin Cancer Res. 2024;43:69.
Liu L, Liu Y, Zhang S, Zhang J, Meng Y, Liu D, et al. Celastrol promotes apoptosis of breast cancer MDA-MB-231 cells by targeting HSDL2. Acupunct Herb Med. 2024;4:92–101.
Qu J, Qiu B, Zhang Y, Hu Y, Wang Z, Guan Z, et al. The tumor-enriched small molecule gambogic amide suppresses glioma by targeting WDR1-dependent cytoskeleton remodeling. Signal Transduct Target Ther. 2023;8:424.
Liu TT, Yang H, Zhuo FF, Yang Z, Zhao MM, Guo Q, et al. Atypical E3 ligase ZFP91 promotes small-molecule-induced E2F2 transcription factor degradation for cancer therapy. EBioMedicine. 2022;86:104353.
Jeon YJ, Khelifa S, Ratnikov B, Scott DA, Feng Y, Parisi F, et al. Regulation of glutamine carrier proteins by RNF5 determines breast cancer response to ER stress-inducing chemotherapies. Cancer Cell. 2015;27:354–69.
Chung CI, Zhang Q, Shu X. Dynamic imaging of small molecule induced protein-protein interactions in living cells with a fluorophore phase transition based approach. Anal Chem. 2018;90:14287–93.
Yu TJ, Ma D, Liu YY, Xiao Y, Gong Y, Jiang YZ, et al. Bulk and single-cell transcriptome profiling reveal the metabolic heterogeneity in human breast cancers. Mol Ther. 2021;29:2350–65.
Sengupta D, Cassel T, Teng KY, Aljuhani M, Chowdhary VK, Hu P, et al. Regulation of hepatic glutamine metabolism by miR-122. Mol Metab. 2020;34:174–86.
Zong WX, Rabinowitz JD, White E. Mitochondria and cancer. Mol Cell. 2016;61:667–76.
Cheng S, Wan X, Yang L, Qin Y, Chen S, Liu Y, et al. RGCC-mediated PLK1 activity drives breast cancer lung metastasis by phosphorylating AMPKalpha2 to activate oxidative phosphorylation and fatty acid oxidation. J Exp Clin Cancer Res. 2023;42:342.
Zhang DX, Zhang JP, Hu JY, Huang YS. The potential regulatory roles of NAD+ and its metabolism in autophagy. Metabolism. 2016;65:454–62.
White AT, Schenk S. NAD+/NADH and skeletal muscle mitochondrial adaptations to exercise. Am J Physiol Endocrinol Metab. 2012;303:E308–21.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Prim. 2020;6:92.
Anto R, Vidya K, Thomas M, Tirkey AJ, Agarwal M, Riju J, et al. Worst pattern of invasion as an independent predictor of lymph node metastasis and prognosis in oral cavity squamous cell carcinoma - a retrospective cohort study. Indian J Otolaryngol Head Neck Surg. 2023;75:440–9.
Cheng X, Feng M, Zhang A, Guo J, Gong Y, Hu X, et al. Gambogenic acid induces apoptosis via upregulation of Noxa in oral squamous cell carcinoma. Chin J Nat Med. 2024;22:632–42.
Allevato MM, Trinh S, Koshizuka K, Nachmanson D, Nguyen TC, Yokoyama Y, et al. A genome-wide CRISPR screen reveals that antagonism of glutamine metabolism sensitizes head and neck squamous cell carcinoma to ferroptotic cell death. Cancer Lett. 2024;598:217089.
Song A, Wu L, Zhang BX, Yang QC, Liu YT, Li H, et al. Glutamine inhibition combined with CD47 blockade enhances radiotherapy-induced ferroptosis in head and neck squamous cell carcinoma. Cancer Lett. 2024;588:216727.
Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–84.
Zhou Q, Lin W, Wang C, Sun F, Ju S, Chen Q, et al. Neddylation inhibition induces glutamine uptake and metabolism by targeting CRL3(SPOP) E3 ligase in cancer cells. Nat Commun. 2022;13:3034.
Nikkuni O, Kaira K, Toyoda M, Shino M, Sakakura K, Takahashi K, et al. Expression of amino acid transporters (LAT1 and ASCT2) in patients with stage III/IV laryngeal squamous cell carcinoma. Pathol Oncol Res. 2015;21:1175–81.
Wen Q, Huang M, Xie J, Liu R, Miao Q, Huang J, et al. lncRNA SYTL5-OT4 promotes vessel co-option by inhibiting the autophagic degradation of ASCT2. Drug Resist Updat. 2023;69:100975.
Jiang H, Zhang N, Tang T, Feng F, Sun H, Qu W. Target the human alanine/serine/cysteine transporter 2(ASCT2): achievement and future for novel cancer therapy. Pharmacol Res. 2020;158:104844.
Li S, Xiong Q, Shen Y, Lin J, Zhang L, Wu Y, et al. Toosendanin: upgrade of an old agent in cancer treatment. Chin J Nat Med. 2024;22:887–99.
Huang D, Tang Z, Pu X, Wang T, Gao F, Li C. A novel cabazitaxel liposomes modified with ginsenoside Rk1 for cancer targeted therapy. Acupunct Herb Med. 2024;4:113–21.
Yang L, Wang Y, Ye X, Liu Q, Qu D, Chen Y. Traditional Chinese medicine-based drug delivery systems for anti-tumor therapies. Chin J Nat Med. 2024;22:1177–92.
Acknowledgements
This work was supported by National Natural Science Foundation of China (No. 82322073, 82173846, 82304790, and 82430119), China Postdoctoral Innovative Talent Support Program (BX20220213), Shanghai Rising-Star Program (No.22QA1409100), Oriental Scholars of Shanghai (TP2022081), Jiangxi Province Thousand Talents Program (jxsq2023102168), Shanghai Shuguang Program (No. 24SG40), National Key R&D Program of China (Grant No. 2024YFC3506600), CAMS Innovation Fund for Medical Sciences (CIFMS) (2023-I2M-3-009), Key project at central government level: The ability establishment of sustainable use for valuable Chinese medicine resources (2060302), Shanghai Sailing Program (No. 22YF1445000, 23YF1442600), High-level Key Discipline of National Administration of Traditional Chinese Medicine (No. zyyzdxk-2023071), Innovation team of high-level local universities in Shanghai: Strategic Innovation Team of TCM Chemical Biology, Organizational Key Research and Development Program of Shanghai University of Traditional Chinese Medicine [No. 2023YZZ02], supported by the State Key Laboratory of Discovery and Utilization of Functional Components in Traditional Chinese Medicine. We thank the staff members of the Large-scale Protein Preparation System (https://cstr.cn/31129.02.NFPS.LSPS) at the National Facility for Protein Science in Shanghai (https://cstr.cn/31129.02.NFPS), for providing technical support and assistance in data collection and analysis. We acknowledge the online drawing software for figure creation (BioRender, https://biorender.com).
Author information
Authors and Affiliations
Contributions
XYC, XC, and XHL: Writing—original draft. DL, QYX, XXL, and JYL: Methodology. RRP: Provision of compounds. LJZ and HZC: Investigation. JMJ, WDZ, and XL: Review & editing and conceptualization.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Xy., Chen, X., Liang, Xh. et al. Yuanhuacine suppresses head and neck cancer growth by promoting ASCT2 degradation and inhibiting glutamine uptake. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01562-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01562-2