Abstract
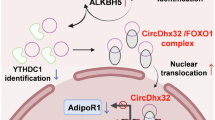
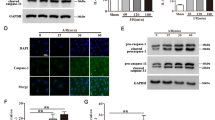
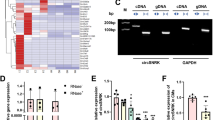
Circular RNAs (circRNAs) are a distinct class of endogenous RNAs characterized by their covalently closed circular structure. CircRNAs play crucial regulatory roles in various biological processes and pathogenesis. In this study we investigated the role of circRNAs in cardiomyocyte pyroptosis and underlying mechanisms. Ischemia/reperfusion (I/R)-induced myocardial injury was induced in mice by ligation of the left anterior descending coronary artery (LAD). Neonatal mouse cardiomyocytes were subjected to hypoxia/reoxygenation (H/R) assault. By using circRNA microarray, we found that the expression levels of a pyroptosis-related circRNA (designated PYRCR) were markedly decreased in H/R-exposed cardiomyocytes and I/R-injured mouse hearts. Overexpression of PYRCR inhibited cardiomyocyte pyroptosis, attenuated I/R-induced myocardial infarction and ameliorated cardiac function in mice. By RNA pull-down assays coupled with MS analysis followed by molecular validation, we identified developmental regulated GTP-binding protein 2 (DRG2) as the direct downstream target of PYRCR. Cardiac-specific DRG2 knockout mice displayed attenuated pyroptosis and enhanced cardiac function following I/R injury compared to DRG2fl/fl controls. DRG2 directly bound to dynamin-related protein 1 (Drp1), the master regulator of mitochondrial fission, and enhanced its protein stability and expression. Importantly, PYRCR competitively disrupted the DRG2-Drp1 interaction, thereby suppressing DRG2-mediated Drp1 expression and subsequently reducing mitochondrial fission, cardiomyocyte pyroptosis, and myocardial damage. In conclusion, we demonstrate that PYRCR, a novel pyroptosis-related circRNA, protects against I/R-induced myocardial injury through the DRG2-mediated modulation of Drp1 activity, offering promising new therapeutic strategies for preventing cardiac damage mediated by cardiomyocyte pyroptosis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
All data generated in the present study may be requested from the corresponding author.
References
Mohebi R, Chen C, Ibrahim NE, McCarthy CP, Gaggin HK, Singer DE, et al. Cardiovascular disease projections in the United States based on the 2020 census estimates. J Am Coll Cardiol. 2022;80:565–78.
Bozkurt B, Ahmad T, Alexander KM, Baker WL, Bosak K, Breathett K, et al. Heart failure epidemiology and outcomes statistics: a report of the heart failure society of America. J Card Fail. 2023;29:1412–51.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143:e254–e743.
Sabolová G, Kočan L, Rabajdová M, Rapčanová S, Vašková J. Association of inflammation, oxidative stress, and deteriorated cognitive functions in patients after cardiac surgery. Vessel Plus. 2024;8:27.
Botros M, Fadah K, Mukherjee D. The role of inflammatory response in the development of atherosclerosis, myocardial infarction, and remodeling. Vessel. 2024;8:31.
Ying L, Benjanuwattra J, Chattipakorn SC, Chattipakorn N. The role of RIPK3-regulated cell death pathways and necroptosis in the pathogenesis of cardiac ischaemia-reperfusion injury. Acta Physiol. 2021;231:e13541.
Ju J, Song YN, Wang K. Mechanism of ferroptosis: a potential target for cardiovascular diseases treatment. Aging Dis. 2021;12:261–76.
Shen S, Wang Z, Sun H, Ma L. Role of NLRP3 inflammasome in myocardial ischemia-reperfusion injury and ventricular remodeling. Med Sci Monit. 2022;28:e934255.
Cookson BT, Brennan MA. Pro-inflammatory programmed cell death. Trends Microbiol. 2001;9:113–4.
Vande Walle L, Lamkanfi M. Pyroptosis. Current Biol CB. 2016;26:R568–r72.
Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 2002;9:358–61.
Kayagaki N, Stowe IB, Lee BL, O’Rourke K, Anderson K, Warming S, et al. Caspase-11 cleaves gasdermin D for non-canonical inflammasome signalling. Nature. 2015;526:666–71.
Schmid-Burgk JL, Gaidt MM, Schmidt T, Ebert TS, Bartok E, Hornung V. Caspase-4 mediates non-canonical activation of the NLRP3 inflammasome in human myeloid cells. Eur J Immunol. 2015;45:2911–7.
Shi J, Zhao Y, Wang K, Shi X, Wang Y, Huang H, et al. Cleavage of GSDMD by inflammatory caspases determines pyroptotic cell death. Nature. 2015;526:660–5.
Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci USA. 1976;73:3852–6.
Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–40.
Zaug AJ, Grabowski PJ, Cech TR. Autocatalytic cyclization of an excised intervening sequence RNA is a cleavage-ligation reaction. Nature. 1983;301:578–83.
Mehta SL, Chokkalla AK, Bathula S, Arruri V, Chelluboina B, Vemuganti R. CDR1as regulates α-synuclein-mediated ischemic brain damage by controlling miR-7 availability. Mol Ther Nucleic Acids. 2023;31:57–67.
Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, et al. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14:361–9.
Patop IL, Wüst S, Kadener S. Past, present, and future of circRNAs. EMBO J. 2019;38:e100836.
Kristensen LS, Andersen MS, Stagsted LVW, Ebbesen KK, Hansen TB, Kjems J. The biogenesis, biology and characterization of circular RNAs. Nat Rev Genet. 2019;20:675–91.
Wang K, Long B, Liu F, Wang JX, Liu CY, Zhao B, et al. A circular RNA protects the heart from pathological hypertrophy and heart failure by targeting miR-223. Eur Heart J. 2016;37:2602–11.
Zeng Y, Du WW, Wu Y, Yang Z, Awan FM, Li X, et al. A circular RNA binds to and activates AKT phosphorylation and nuclear localization reducing apoptosis and enhancing cardiac repair. Theranostics. 2017;7:3842–55.
Du WW, Yang W, Chen Y, Wu ZK, Foster FS, Yang Z, et al. Foxo3 circular RNA promotes cardiac senescence by modulating multiple factors associated with stress and senescence responses. Eur Heart J. 2017;38:1402–12.
Schenker T, Lach C, Kessler B, Calderara S, Trueb B. A novel GTP-binding protein which is selectively repressed in SV40 transformed fibroblasts. J Biol Chem. 1994;269:25447–53.
Li B, Trueb B. DRG represents a family of two closely related GTP-binding proteins. Biochim Biophys Acta. 2000;1491:196–204.
Mani M, Thao DT, Kim BC, Lee UH, Kim DJ, Jang SH, et al. DRG2 knockdown induces Golgi fragmentation via GSK3β phosphorylation and microtubule stabilization. Biochim Biophys Acta Mol Cell Res. 2019;1866:1463–74.
Vo MT, Ko MS, Lee UH, Yoon EH, Lee BJ, Cho WJ, et al. Developmentally regulated GTP-binding protein 2 depletion leads to mitochondrial dysfunction through downregulation of dynamin-related protein 1. Biochem Biophys Res Commun. 2017;486:1014–20.
Mani M, Lee UH, Yoon NA, Yoon EH, Lee BJ, Cho WJ, et al. Developmentally regulated GTP-binding protein 2 is required for stabilization of Rac1-positive membrane tubules. Biochem Biophys Res Commun. 2017;493:758–64.
Yoon NA, Jung SJ, Choi SH, Ryu JH, Mani M, Lee UH, et al. DRG2 supports the growth of primary tumors and metastases of melanoma by enhancing VEGF-A expression. FEBS J. 2020;287:2070–86.
Lim HR, Vo MT, Kim DJ, Lee UH, Yoon JH, Kim HJ, et al. DRG2 deficient mice exhibit impaired motor behaviors with reduced striatal dopamine release. Int J Mol Sci. 2019;21:60.
Le AN, Park SS, Le MX, Lee UH, Ko BK, Lim HR, et al. DRG2 depletion promotes endothelial cell senescence and vascular endothelial dysfunction. Int J Mol Sci. 2022;23:2877.
Lee GJ, Yan L, Vatner DE, Vatner SF. Mst1 inhibition rescues beta1-adrenergic cardiomyopathy by reducing myocyte necrosis and non-myocyte apoptosis rather than myocyte apoptosis. Basic Res Cardiol. 2015;110:7.
Duan Y, Li Q, Wu J, Zhou C, Liu X, Yue J, et al. A detrimental role of endothelial S1PR2 in cardiac ischemia-reperfusion injury via modulating mitochondrial dysfunction, NLRP3 inflammasome activation, and pyroptosis. Redox Biol. 2024;75:103244.
Zeng C, Duan F, Hu J, Luo B, Huang B, Lou X, et al. NLRP3 inflammasome-mediated pyroptosis contributes to the pathogenesis of non-ischemic dilated cardiomyopathy. Redox Biol. 2020;34:101523.
Guo YW, Zhu L, Duan YT, Hu YQ, Li LB, Fan WJ, et al. Ruxolitinib induces apoptosis and pyroptosis of anaplastic thyroid cancer via the transcriptional inhibition of DRP1-mediated mitochondrial fission. Cell Death Dis. 2024;15:125.
Yang B, Wang J, Qiao J, Zhang Q, Liu Q, Tan Y, et al. Circ DENND4C inhibits pyroptosis and alleviates ischemia-reperfusion acute kidney injury by exosomes secreted from human urine-derived stem cells. Chem Biol Interact. 2024;391:110922.
Yang D, Zhao D, Ji J, Wang C, Liu N, Bao X, et al. CircRNA_0075723 protects against pneumonia-induced sepsis through inhibiting macrophage pyroptosis by sponging miR-155-5p and regulating SHIP1 expression. Front Immunol. 2023;14:1095457.
Xu S, Ge Y, Wang X, Yin W, Zhu X, Wang J, et al. Circ-USP9X interacts with EIF4A3 to promote endothelial cell pyroptosis by regulating GSDMD stability in atherosclerosis. Clin Exp Hypertens. 2023;45:2186319.
Yuan Q, Sun Y, Yang F, Yan D, Shen M, Jin Z, et al. CircRNA DICAR as a novel endogenous regulator for diabetic cardiomyopathy and diabetic pyroptosis of cardiomyocytes. Sig Transduct Target Ther. 2023;8:99.
Xu C, Li H, Zhang L, Jia T, Duan L, Lu C. MicroRNA‑1915‑3p prevents the apoptosis of lung cancer cells by downregulating DRG2 and PBX2. Mol Med Rep. 2016;13:505–12.
Kim SC, Lee WH, Kim SH, Abdulkhayevich AA, Park JW, Kim YM, et al. Developmentally regulated GTP-binding protein 2 levels in prostate cancer cell lines impact docetaxel-induced apoptosis. Investig Clin Urol. 2021;62:485–95.
Quiles JM, Gustafsson ÅB. The role of mitochondrial fission in cardiovascular health and disease. Nat Rev Cardiol. 2022;19:723–36.
Acknowledgements
We thank Prof. Chen Chen and Prof. Dao-wen Wang from Tongji Hospital, Huazhong University of Science and Technology, for providing samples from patients with heart failure and healthy subjects. The mechanism diagram in the article: “By figdraw.com”.
Funding
This work was supported by the National Natural Science Foundation of China (82370291, 82401019, 82270288), the Open Project Program of State Key Laboratory of Frigid Zone Cardiovascular Diseases (SKLFZCD), Harbin Medical University (HDHY2024007), Qingdao Science and Technology Benefiting the People Demonstration Project (24-1-8-smjk-7-nsh), Major Basic Research Projects in Shandong Province (ZR2024ZD46), Taishan Scholar Distinguished Expert, China Postdoctoral Science Foundation (2024M761553) and Shandong Provincial Natural Science Foundation (ZR2024QH184).
Author information
Authors and Affiliations
Contributions
KW, XQG, YHZ, and FL designed and supervised the experiments. XZC, HFX, XMZ, JHR, FHL and LYZ performed the experiments. CYL, YQW, SMY, XZC and HFX analyzed the data. XQG, KW, YHZ and XZC wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All the animal studies conformed to Directive 2010/63/EU of the European Parliament and were approved by the Biomedical Research Ethics Committee of Qingdao University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Xz., Xu, Hf., Zhao, Xm. et al. PYRCR alleviates myocardial ischemia/reperfusion injury in mice via inhibiting DRG2-mediated cardiomyocyte pyroptosis. Acta Pharmacol Sin (2025). https://doi.org/10.1038/s41401-025-01604-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41401-025-01604-9