Abstract
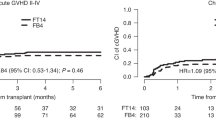
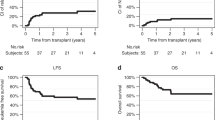
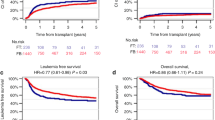
Reduced-toxicity conditioning (RTC) regimens aim to mitigate regimen-related toxicity while maintaining anti-leukemic efficacy in allogeneic hematopoietic stem cell transplantation (allo-HSCT). We assessed outcomes of RTC regimens utilizing melphalan versus intravenous busulfan combined with fludarabine in adult acute lymphoblastic leukemia (ALL) patients. A retrospective analysis was conducted with 149 consecutive adult ALL patients (median age 51, range 18–60) in remission undergoing allo-HSCT. Patients received either fludarabine 150 mg/BSA plus 2 days of melphalan 70 mg/BSA (FM140, n = 76) from 2009 to 2015 or fludarabine plus 3 days of busulfan 3.2 mg/kg (FB9.6, n = 73) from 2016 to 2021. At 5 years post-HSCT, FM140 demonstrated superior disease-free survival (53.4% vs. 30.5%, p = 0.007) and lower cumulative relapse (27.4% vs. 46.8%, p = 0.026) than FB9.6. Five-year overall survival and non-relapse mortality did not significantly differ. FM140 exhibited a higher incidence of acute graft-versus-host disease (GVHD) grades II-IV (49.3% vs. 30.3%, p = 0.016), though rates of acute GVHD grades III-IV and chronic GVHD were similar. Multivariate analysis identified Philadelphia chromosome and minimal residual disease positive status, and FB9.6 conditioning as predictors of increased relapse and poorer disease-free survival. FM140 RTC regimen displayed significantly reduced relapse and superior disease-free survival compared to FB9.6 in ALL patients undergoing allo-HSCT, highlighting its current clinical utility.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–26. https://doi.org/10.1056/NEJMra052638
Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biol Blood Marrow Transpl. 2009;15:1628–33. https://doi.org/10.1016/j.bbmt.2009.07.004.
Santarone S, Pidala J, Di Nicola M, Field T, Alsina M, Ayala E, et al. Fludarabine and pharmacokinetic-targeted busulfan before allografting for adults with acute lymphoid leukemia. Biol Blood Marrow Transpl. 2011;17:1505–11. https://doi.org/10.1016/j.bbmt.2011.02.011.
Spyridonidis A, Labopin M, Savani B, Giebel S, Bug G, Schönland S, et al. Reduced 8-gray compared to standard 12-gray total body irradiation for allogeneic transplantation in first remission acute lymphoblastic leukemia: a study of the acute leukemia working party of the EBMT. Hemasphere. 2023;7:e812. https://doi.org/10.1097/hs9.0000000000000812.
Gagelmann N, Kroger N. Dose intensity for conditioning in allogeneic hematopoietic cell transplantation: can we recommend “when and for whom” in 2021? Haematologica. 2021;106:1794–804. https://doi.org/10.3324/haematol.2020.268839.
Spyridonidis A, Labopin M, Savani BN, Niittyvuopio R, Blaise D, Craddock C, et al. Redefining and measuring transplant conditioning intensity in current era: a study in acute myeloid leukemia patients. Bone Marrow Transpl. 2020;55:1114–25. https://doi.org/10.1038/s41409-020-0803-y.
Marks R, Potthoff K, Hahn J, Ihorst G, Bertz H, Spyridonidis A, et al. Reduced-toxicity conditioning with fludarabine, BCNU, and melphalan in allogeneic hematopoietic cell transplantation: particular activity against advanced hematologic malignancies. Blood. 2008;112:415–25. https://doi.org/10.1182/blood-2007-08-104745.
Hirschbuhl K, Labopin M, Polge E, Blaise D, Bourhis JH, Socie G, et al. Total body irradiation versus busulfan based intermediate intensity conditioning for stem cell transplantation in ALL patients >45 years-a registry-based study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2023;58:874–80. https://doi.org/10.1038/s41409-023-01966-w.
Giebel S, Labopin M, Sobczyk-Kruszelnicka M, Stelljes M, Byrne JL, Fegueux N, et al. Total body irradiation + fludarabine compared to busulfan + fludarabine as “reduced-toxicity conditioning” for patients with acute myeloid leukemia treated with allogeneic hematopoietic cell transplantation in first complete remission: a study by the Acute Leukemia Working Party of the EBMT. Bone Marrow Transpl. 2021;56:481–91. https://doi.org/10.1038/s41409-020-01050-7.
Malard F, Mohty M. Acute lymphoblastic leukaemia. Lancet. 2020;395:1146–62. https://doi.org/10.1016/S0140-6736(19)33018-1
Malard F, Chevallier P, Guillaume T, Delaunay J, Rialland F, Harousseau JL, et al. Continuous reduced nonrelapse mortality after allogeneic hematopoietic stem cell transplantation: a single-institution’s three decade experience. Biol Blood Marrow Transpl. 2014;20:1217–23. https://doi.org/10.1016/j.bbmt.2014.04.021.
Peric Z, Labopin M, Peczynski C, Polge E, Cornelissen J, Carpenter B, et al. Comparison of reduced-intensity conditioning regimens in patients with acute lymphoblastic leukemia >45 years undergoing allogeneic stem cell transplantation-a retrospective study by the Acute Leukemia Working Party of EBMT. Bone Marrow Transpl. 2020;55:1560–9. https://doi.org/10.1038/s41409-020-0878-5.
Jain T, Alahdab F, Firwana B, Sonbol MB, Almader-Douglas D, Palmer J. Choosing a reduced-intensity conditioning regimen for allogeneic stem cell transplantation, fludarabine/busulfan versus fludarabine melphalan: a systematic review and meta-analysis. Biol Blood Marrow Transpl. 2019;25:728–33. https://doi.org/10.1016/j.bbmt.2018.11.016.
Yoon JH, Min GJ, Park SS, Jeon YW, Lee SE, Cho BS, et al. Minimal residual disease-based long-term efficacy of reduced-intensity conditioning versus myeloablative conditioning for adult Philadelphia-positive acute lymphoblastic leukemia. Cancer. 2019;125:873–83. https://doi.org/10.1002/cncr.31874.
Eom KS, Shin SH, Yoon JH, Yahng SA, Lee SE, Cho BS, et al. Comparable long-term outcomes after reduced-intensity conditioning versus myeloablative conditioning allogeneic stem cell transplantation for adult high-risk acute lymphoblastic leukemia in complete remission. Am J Hematol. 2013;88:634–41. https://doi.org/10.1002/ajh.23465.
Lee S, Kim DW, Cho BS, Yoon JH, Shin SH, Yahng SA, et al. Impact of minimal residual disease kinetics during imatinib-based treatment on transplantation outcome in Philadelphia chromosome-positive acute lymphoblastic leukemia. Leukemia. 2012;26:2367–74. https://doi.org/10.1038/leu.2012.164.
Yoon JH, Yhim HY, Kwak JY, Ahn JS, Yang DH, Lee JJ, et al. Minimal residual disease-based effect and long-term outcome of first-line dasatinib combined with chemotherapy for adult Philadelphia chromosome-positive acute lymphoblastic leukemia. Ann Oncol. 2016;27:1081–8. https://doi.org/10.1093/annonc/mdw123
Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, et al. Feasible outcome of blinatumomab followed by allogeneic hematopoietic cell transplantation for adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first salvage. Cancer Med. 2019;8:7650–9. https://doi.org/10.1002/cam4.2680.
Yoon SY, Yoon JH, Min GJ, Park SS, Park S, Lee SE, et al. Experience of blinatumomab salvage for patients with acute lymphoblastic leukemia presenting with isolated extramedullary relapse after previous allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2020;55:1469–72. https://doi.org/10.1038/s41409-019-0708-9.
Lee SH, Yoon JH, Min GJ, Park SS, Park S, Lee SE, et al. Response to blinatumomab or inotuzumab ozogamicin for isolated extramedullary relapse of adult acute lymphoblastic leukemia after allogeneic hematopoietic cell transplantation: a case study. Int J Hematol. 2022;115:135–9. https://doi.org/10.1007/s12185-021-03231-6.
Yoon JH, Kwag D, Lee JH, Min GJ, Park SS, Park S, et al. Superior survival outcome of blinatumomab compared with conventional chemotherapy for adult patients with relapsed or refractory B-cell precursor acute lymphoblastic leukemia: a propensity score-matched cohort analysis. Ther Adv Hematol. 2023;14:20406207231154713 https://doi.org/10.1177/20406207231154713.
Mohty M, Labopin M, Volin L, Gratwohl A, Socie G, Esteve J, et al. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116:4439–43. https://doi.org/10.1182/blood-2010-02-266551.
Kawamura K, Kako S, Mizuta S, Ishiyama K, Aoki J, Yano S, et al. Comparison of conditioning with fludarabine/busulfan and fludarabine/melphalan in allogeneic transplantation recipients 50 years or older. Biol Blood Marrow Transpl. 2017;23:2079–87. https://doi.org/10.1016/j.bbmt.2017.09.003.
Yoon JH, Min GJ, Park SS, Park S, Lee SE, Cho BS, et al. Impact of donor type on long-term graft-versus-host disease-free/relapse-free survival for adult acute lymphoblastic leukemia in first remission. Bone Marrow Transpl. 2021;56:828–40. https://doi.org/10.1038/s41409-020-01097-6.
Malard F, Holler E, Sandmaier BM, Huang H, Mohty M. Acute graft-versus-host disease. Nat Rev Dis Prim. 2023;9:27 https://doi.org/10.1038/s41572-023-00438-1.
Velardi E, Tsai JJ, van den Brink MRM. T cell regeneration after immunological injury. Nat Rev Immunol. 2021;21:277–91. https://doi.org/10.1038/s41577-020-00457-z.
Spyridonidis A, Labopin M, Gedde-Dahl T, Ganser A, Stelljes M, Craddock C, et al. Validation of the transplant conditioning intensity (TCI) index for allogeneic hematopoietic cell transplantation. Bone Marrow Transpl. 2024;59:217–23. https://doi.org/10.1038/s41409-023-02139-5.
Shimoni A, Hardan I, Shem-Tov N, Rand A, Herscovici C, Yerushalmi R, et al. Comparison between two fludarabine-based reduced-intensity conditioning regimens before allogeneic hematopoietic stem-cell transplantation: fludarabine/melphalan is associated with higher incidence of acute graft-versus-host disease and non-relapse mortality and lower incidence of relapse than fludarabine/busulfan. Leukemia. 2007;21:2109–16. https://doi.org/10.1038/sj.leu.2404886.
Short NJ, Kantarjian H, Jabbour E. Optimizing the treatment of acute lymphoblastic leukemia in younger and older adults: new drugs and evolving paradigms. Leukemia. 2021;35:3044–58. https://doi.org/10.1038/s41375-021-01277-3.
Teachey DT, Hunger SP. Acute lymphoblastic leukaemia in 2017: Immunotherapy for ALL takes the world by storm. Nat Rev Clin Oncol. 2018;15:69–70. https://doi.org/10.1038/nrclinonc.2017.176.
van den Brink MR, Velardi E, Perales MA. Immune reconstitution following stem cell transplantation. Hematol Am Soc Hematol Educ Program. 2015;2015:215–9. https://doi.org/10.1182/asheducation-2015.1.215
Mensen A, Johrens K, Anagnostopoulos I, Demski S, Oey M, Stroux A, et al. Bone marrow T-cell infiltration during acute GVHD is associated with delayed B-cell recovery and function after HSCT. Blood. 2014;124:963–72. https://doi.org/10.1182/blood-2013-11-539031.
Troullioud Lucas AG, Lindemans CA, Bhoopalan SV, Dandis R, Prockop SE, Naik S, et al. Early immune reconstitution as predictor for outcomes after allogeneic hematopoietic cell transplant; a tri-institutional analysis. Cytotherapy. 2023;25:977–85. https://doi.org/10.1016/j.jcyt.2023.05.012.
Kuczma M, Ding ZC, Zhou G. Immunostimulatory effects of melphalan and usefulness in adoptive cell therapy with antitumor CD4+ T cells. Crit Rev Immunol. 2016;36:179–91. https://doi.org/10.1615/CritRevImmunol.2016017507
Kim HT, Armand P, Frederick D, Andler E, Cutler C, Koreth J, et al. Absolute lymphocyte count recovery after allogeneic hematopoietic stem cell transplantation predicts clinical outcome. Biol Blood Marrow Transpl. 2015;21:873–80. https://doi.org/10.1016/j.bbmt.2015.01.019.
Federmann B, Bornhauser M, Meisner C, Kordelas L, Beelen DW, Stuhler G, et al. Haploidentical allogeneic hematopoietic cell transplantation in adults using CD3/CD19 depletion and reduced intensity conditioning: a phase II study. Haematologica. 2012;97:1523–31. https://doi.org/10.3324/haematol.2011.059378.
Pastore D, Delia M, Mestice A, Carluccio P, Perrone T, Gaudio F, et al. CD3+/Tregs ratio in donor grafts is linked to acute graft-versus-host disease and immunologic recovery after allogeneic peripheral blood stem cell transplantation. Biol Blood Marrow Transpl. 2012;18:887–93. https://doi.org/10.1016/j.bbmt.2011.10.039.
Divito SJ, Aasebo AT, Matos TR, Hsieh PC, Collin M, Elco CP, et al. Peripheral host T cells survive hematopoietic stem cell transplantation and promote graft-versus-host disease. J Clin Invest. 2020;130:4624–36. https://doi.org/10.1172/JCI129965
Penack O, Marchetti M, Ruutu T, Aljurf M, Bacigalupo A, Bonifazi F, et al. Prophylaxis and management of graft versus host disease after stem-cell transplantation for haematological malignancies: updated consensus recommendations of the European Society for Blood and Marrow Transplantation. Lancet Haematol. 2020;7:e157–e167. https://doi.org/10.1016/S2352-3026(19)30256-X
Baron F, Mohty M, Blaise D, Socié G, Labopin M, Esteve J, et al. Anti-thymocyte globulin as graft-versus-host disease prevention in the setting of allogeneic peripheral blood stem cell transplantation: a review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica. 2017;102:224–34. https://doi.org/10.3324/haematol.2016.148510.
Bonifazi F, Rubio MT, Bacigalupo A, Boelens JJ, Finke J, Greinix H, et al. Rabbit ATG/ATLG in preventing graft-versus-host disease after allogeneic stem cell transplantation: consensus-based recommendations by an international expert panel. Bone Marrow Transpl. 2020;55:1093–102. https://doi.org/10.1038/s41409-020-0792-x.
Massoud R, Klyuchnikov E, Gagelmann N, Zabelina T, Wolschke C, Ayuk F, et al. Impact of Anti-T-lymphocyte globulin dosing on GVHD and Immune reconstitution in matched unrelated myeloablative peripheral blood stem cell transplantation. Bone Marrow Transpl. 2022;57:1548–55. https://doi.org/10.1038/s41409-022-01666-x.
Heining C, Spyridonidis A, Bernhardt E, Schulte-Monting J, Behringer D, Grullich C, et al. Lymphocyte reconstitution following allogeneic hematopoietic stem cell transplantation: a retrospective study including 148 patients. Bone Marrow Transpl. 2007;39:613–22. https://doi.org/10.1038/sj.bmt.1705648.
Hill GR, Crawford JM, Cooke KR, Brinson YS, Pan L, Ferrara JLM. Total body irradiation and acute graft-versus-host disease: the role of gastrointestinal damage and inflammatory cytokines. Blood. 1997;90:3204–13. https://doi.org/10.1182/blood.V90.8.3204
Antin JH, Ferrara JL. Cytokine dysregulation and acute graft-versus-host disease. Blood. 1992;80:2964–8. https://doi.org/10.1182/blood.V80.12.2964.2964
Johansson JE, Brune M, Ekman T. The gut mucosa barrier is preserved during allogeneic, haemopoietic stem cell transplantation with reduced intensity conditioning. Bone Marrow Transpl. 2001;28:737–42. https://doi.org/10.1038/sj.bmt.1703230
Kekre N, Marquez-Malaver FJ, Cabrero M, Pinana J, Esquirol A, Soiffer RJ, et al. Fludarabine/busulfan versus fludarabine/melphalan conditioning in patients undergoing reduced-intensity conditioning hematopoietic stem cell transplantation for lymphoma. Biol Blood Marrow Transpl. 2016;22:1808–15. https://doi.org/10.1016/j.bbmt.2016.07.006.
Yamada Y, Ikegawa S, Najima Y, Atsuta Y, Konuma R, Adachi H, et al. Retrospective comparison of hematopoietic stem cell transplantation following reduced-intensity conditioning with fludarabine/low-dose melphalan plus 4 Gy TBI versus fludarabine/ busulfan plus 4 Gy TBI. Int J Hematol. 2022;115:244–54. https://doi.org/10.1007/s12185-021-03233-4.
Bayraktar UD, Bashir Q, Qazilbash M, Champlin RE, Ciurea SO. Fifty years of melphalan use in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2013;19:344–56. https://doi.org/10.1016/j.bbmt.2012.08.011.
Piñana JL, Perez-Pitarch A, Garcia-Cadenas I, Barba P, Hernandez-Boluda JC, Esquirol A, et al. A time-to-event model for acute kidney injury after reduced-intensity conditioning stem cell transplantation using a tacrolimus- and sirolimus-based graft-versus-host disease prophylaxis. Biol Blood Marrow Transpl. 2017;23:1177–85. https://doi.org/10.1016/j.bbmt.2017.03.035.
Vergara-Cadavid J, Johnson PC, Kim HT, Yi A, Sise ME, Leaf DE et al. Clinical features of acute kidney injury in the early post-transplantation period following reduced-intensity conditioning allogeneic hematopoietic stem cell transplantation. Transplant Cell Ther. 2023. https://doi.org/10.1016/j.jtct.2023.03.029
Ciurea SO, Andersson BS. Busulfan in hematopoietic stem cell transplantation. Biol Blood Marrow Transpl. 2009;15:523–36. https://doi.org/10.1016/j.bbmt.2008.12.489.
Kashyap A, Wingard J, Cagnoni P, Roy J, Tarantolo S, Hu W, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transpl. 2002;8:493–500. https://doi.org/10.1053/bbmt.2002.v8.pm12374454
Vassal G, Deroussent A, Hartmann O, Challine D, Benhamou E, Valteau-Couanet D, et al. Dose-dependent neurotoxicity of high-dose busulfan in children: a clinical and pharmacological study. Cancer Res. 1990;50:6203–7.
Fein JA, Shimoni A, Labopin M, Shem-Tov N, Yerushalmi R, Magen H, et al. The impact of individual comorbidities on non-relapse mortality following allogeneic hematopoietic stem cell transplantation. Leukemia. 2018;32:1787–94. https://doi.org/10.1038/s41375-018-0185-y.
Cahu X, Labopin M, Giebel S, Aljurf M, Kyrcz-Krzemien S, Socié G, et al. Impact of conditioning with TBI in adult patients with T-cell ALL who receive a myeloablative allogeneic stem cell transplantation: a report from the acute leukemia working party of EBMT. Bone Marrow Transpl. 2016;51:351–7. https://doi.org/10.1038/bmt.2015.278.
Chen GL, Hahn T, Wilding GE, Groman A, Hutson A, Zhang Y, et al. Reduced-intensity conditioning with fludarabine, melphalan, and total body irradiation for allogeneic hematopoietic cell transplantation: the effect of increasing melphalan dose on underlying disease and toxicity. Biol Blood Marrow Transpl. 2019;25:689–98. https://doi.org/10.1016/j.bbmt.2018.09.042.
Acknowledgements
We thank all patients and their families for their participation in the trial.
Author information
Authors and Affiliations
Contributions
JA and JHY conceptualized and designed the study, contributed to statistical analysis and data interpretation, and wrote the manuscript as a first author. DHK, GJM, SSP, SP, SEL, BSC, KSE, YJK, HJK, CKM, SGC, provided patients and reviewed this manuscript; SL designed and conducted the study, provided patients and materials, analyzed data, and wrote the manuscript; and all authors reviewed and gave final approval of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
This study was conducted in accordance with the principles of the Declaration of Helsinki and was approved by the Institutional Review Board of the Catholic University of Korea (reference number: KC24RISI0192). Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ahn, J., Yoon, JH., Kwag, D. et al. Comparative analysis of reduced toxicity conditioning regimens between fludarabine plus melphalan and fludarabine plus busulfex in adult patients with acute lymphoblastic leukemia. Bone Marrow Transplant 59, 1413–1422 (2024). https://doi.org/10.1038/s41409-024-02363-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-024-02363-7