Abstract
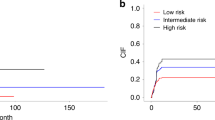
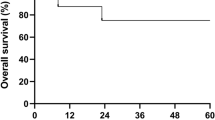
Mixed chimerism (MC) frequently arises in children with severe aplastic anemia (SAA) after allogeneic hematopoietic stem cell transplantation (allo-HSCT). Nonetheless, there is a paucity of research regarding potential predictors and effective interventions. This retrospective study, performed on 150 pediatric patients with SAA who underwent allo-HSCT between December 2015 and June 2022, explored the characteristics, risk factors, treatment, and prognosis of MC. A total of 29 patients (19.3%) developed MC following allo-HSCT, with two individuals experiencing MC twice. The CTX + ATG regimen was associated with the development of MC. Peripheral blood (PB) + bone marrow (BM) stem cell graft and a high number of CD34+ cells were identified as independent protective factors for MC. The cumulative incidence of grade II-IV acute graft-versus-host disease was significantly elevated in donor chimerism (DC) relative to MC. Among MC patients with cytopenia, only two patients who received increased immunosuppression alone were effective. Complete DC was achieved in all four patients who received the second transplantation. In conclusion, we emphasize that prompt second transplantation is essential when cellular therapy and enhanced immunosuppression fail for MC patients with cytopenia.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
Data will be made available on request.
References
Gale RP, Hinterberger W, Young NS, Gennery AR, Dvorak CC, Hebert KM, et al. What causes aplastic anaemia? Leukemia. 2023;37:1191–3.
Young NS. Aplastic Anemia. N. Engl J Med. 2018;379:1643–56.
Solimando AG, Palumbo C, Pragnell MV, Bittrich M, Argentiero A, Krebs M. Aplastic anemia as a roadmap for bone marrow failure: an overview and a clinical workflow. Int J Mol Sci. 2022;23:11765.
Babushok DV, DeZern AE, de Castro CM, Rogers ZR, Beenhouwer D, Broder MS, et al. Modified Delphi panel consensus recommendations for management of severe aplastic anemia. Blood Adv. 2024;8:3946–60.
Montoro J, Eikema DJ, Tuffnell J, Potter V, Kalwak K, Halkes CJM, et al. Alternative donor transplantation for severe aplastic anemia: a comparative study of the SAAWP EBMT. Blood. 2024;144:323–33.
DeZern AE, Zahurak M, Jones RJ, Brodsky RA. Uniform conditioning regardless of donor in bone marrow transplantation for severe aplastic anemia. Haematologica. 2024;109:657–60.
Miura S, Ueda K, Minakawa K, Nollet KE, Ikeda K. Prospects and potential for chimerism analysis after allogeneic hematopoietic stem cell transplantation. Cells. 2024;13:993.
Bader P. Documentation of Engraftment and Chimerism After HSCT. In: Carreras E, Dufour C, Mohty M, Kröger N, editors. The EBMT Handbook: Hematopoietic Stem Cell Transplantation and Cellular Therapies [Internet]. 7th ed. Cham (CH): Springer; 2019 [cited 2024 Sep 4]. Available from: https://www.ncbi.nlm.nih.gov/books/NBK553992/.
Sun Q, Wu B, Zhu Z, Sun C, Xu J, Long H, et al. Allogeneic hematopoietic stem cell transplant for severe aplastic anemia: current state and future directions. Curr Stem Cell Res Ther. 2018;13:350–5.
Verkamp B, Jodele S, Sabulski A, Marsh RA, Kieser P, Jordan MB. Emapalumab therapy for hemophagocytic lymphohistiocytosis prior to reduced-intensity transplantation improves chimerism. Blood. 2024;blood.2024025977.
Zimmerman C, Shenoy S. Chimerism in the realm of hematopoietic stem cell transplantation for non-malignant disorders-a perspective. Front Immunol. 2020;11:1791.
DeFilipp Z, Hefazi M, Chen YB, Blazar BR. Emerging approaches to improve allogeneic hematopoietic cell transplantation outcomes for nonmalignant diseases. Blood. 2022;139:3583–93.
Camitta BM, Storb R, Thomas ED. Aplastic anemia (second of two parts): pathogenesis, diagnosis, treatment, and prognosis. N. Engl J Med. 1982;306:712–8.
Wong RSM, Ho Jang J, Wong LLL, Kim JS, Rojnuckarin P, Goh YT, et al. Monitoring and treatment of paroxysmal nocturnal hemoglobinuria in patients with aplastic anemia in Asia: An expert consensus. Int J Mol Sci. 2024;25:12160.
Xu L, Chen H, Chen J, Han M, Huang H, Lai Y, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China—recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11:33.
Chinese Society of Hematology, Chinese Medical Association. [The consensus of allogeneic hematopoietic transplantation for hematological diseases in China (2014)--indication, conditioning regimen and donor selection]. Zhonghua Xue Ye Xue Za Zhi. 2014;35:775–80.
Przepiorka D, Weisdorf D, Martin P, Klingemann HG, Beatty P, Hows J, et al. 1994 consensus conference on acute GVHD Grading. Bone Marrow Transpl. 1995;15:825–8.
Atkinson K, Horowitz MM, Gale RP, Lee MB, Rimm AA, Bortin MM. Consensus among bone marrow transplanters for diagnosis, grading and treatment of chronic graft-versus-host disease. Committee of the International Bone Marrow Transplant Registry. Bone Marrow Transpl. 1989;4:247–54.
McCann S, Passweg J, Bacigalupo A, Locasciulli A, Locatelli F, Ryan J, et al. The influence of cyclosporin alone, or cyclosporin and methotrexate, on the incidence of mixed haematopoietic chimaerism following allogeneic sibling bone marrow transplantation for severe aplastic anaemia. Bone Marrow Transpl. 2007;39:109–14.
Lawler M, McCann SR, Marsh JCW, Ljungman P, Hows J, Vandenberghe E, et al. Serial chimerism analyses indicate that mixed haemopoietic chimerism influences the probability of graft rejection and disease recurrence following allogeneic stem cell transplantation (SCT) for severe aplastic anaemia (SAA): indication for routine assessment of chimerism post SCT for SAA. Br J Haematol. 2009;144:933–45.
Quiroga M, Pereira NF, Bitencourt MA, Bonfim C, Monteiro MG, de M, Pasquini R. Late chimerical status after bone marrow transplantation in severe aplastic anemia according to two different preparatory regimens. Hematol Transfus Cell Ther. 2018;40:112–9.
Kako S, Yamazaki H, Ohashi K, Ozawa Y, Ota S, Kanda Y, et al. Mixed chimerism and secondary graft failure in allogeneic hematopoietic stem cell transplantation for aplastic anemia. Biol Blood Marrow Transpl. 2020;26:445–50.
Zhang Y, Li Y, Wu L, Zhou M, Wang C, Mo W, et al. Mixed chimerism after allogeneic hematopoietic stem cell transplantation for severe aplastic anemia. Hematology. 2021;26:435–43.
Svenberg P, Mattsson J, Ringdén O, Uzunel M. Allogeneic hematopoietic SCT in patients with non-malignant diseases, and importance of chimerism. Bone Marrow Transpl. 2009;44:757–63.
Ozyurek E, Cowan MJ, Koerper MA, Baxter-Lowe LA, Dvorak CC, Horn BN. Increasing mixed chimerism and the risk of graft loss in children undergoing allogeneic hematopoietic stem cell transplantation for non-malignant disorders. Bone Marrow Transpl. 2008;42:83–91.
Nickel RS, Flegel WA, Adams SD, Hendrickson JE, Liang H, Tisdale JF, et al. The impact of pre-existing HLA and red blood cell antibodies on transfusion support and engraftment in sickle cell disease after nonmyeloablative hematopoietic stem cell transplantation from HLA-matched sibling donors: A prospective, single-center, observational study. EClinicalMedicine. 2020;24:100432.
Kudek MR, Shanley R, Zantek ND, McKenna DH, Smith AR, Miller WP. Impact of graft-recipient ABO compatibility on outcomes after umbilical cord blood transplant for nonmalignant disease. Biol Blood Marrow Transpl. 2016;22:2019–24.
Chen H, Li XY, Zhan LP, Fang JP, Huang K, Li Y, et al. Prediction, management, and prognosis of mixed chimerism after hematopoietic stem cell transplantation in transfusion-dependent pediatric thalassemia patients. Pediatr Transpl. 2020;24:e13876.
Handgretinger R, Arendt AM, Maier CP, Lang P. Ex vivo and in vivo T-cell depletion in allogeneic transplantation: towards less or non-cytotoxic conditioning regimens. Expert Rev Clin Immunol. 2022;18:1285–96.
Xu ZL, Cheng YF, Zhang YY, Mo XD, Han TT, Wang FR, et al. The incidence, clinical outcome, and protective factors of mixed chimerism following hematopoietic stem cell transplantation for severe aplastic anemia. Clin Transpl. 2021;35:e14160.
Rao K, Adams S, Qasim W, Allwood Z, Worth A, Silva J, et al. Effect of stem cell source on long-term chimerism and event-free survival in children with primary immunodeficiency disorders after fludarabine and melphalan conditioning regimen. J Allergy Clin Immunol. 2016;138:1152–60.
Sugita J, Tanaka J, Hashimoto A, Shiratori S, Yasumoto A, Wakasa K, et al. Influence of conditioning regimens and stem cell sources on donor-type chimerism early after stem cell transplantation. Ann Hematol. 2008;87:1003–8.
Iftikhar R, DeFilipp Z, DeZern AE, Pulsipher MA, Bejanyan N, Burroughs LM, et al. Allogeneic hematopoietic cell transplantation for the treatment of severe aplastic anemia: evidence-based guidelines from the American Society for Transplantation and Cellular Therapy. Transpl Cell Ther. 2024;30:1155–70.
Carvallo C, Geller N, Kurlander R, Srinivasan R, Mena O, Igarashi T, et al. Prior chemotherapy and allograft CD34+ dose impact donor engraftment following nonmyeloablative allogeneic stem cell transplantation in patients with solid tumors. Blood. 2004;103:1560–3.
Baron F, Maris MB, Storer BE, Sandmaier BM, Panse JP, Chauncey TR, et al. High doses of transplanted CD34+ cells are associated with rapid T-cell engraftment and lessened risk of graft rejection, but not more graft-versus-host disease after nonmyeloablative conditioning and unrelated hematopoietic cell transplantation. Leukemia. 2005;19:822–8.
Gonzalez-Vicent M, Diaz MA. Higher doses of CD34+ PBPC are associated with a rapid acquisition of full donor chimerism and lower risk of relapse after allogeneic transplantation in pediatric patients with hematological malignancies. J Pediatr Hematol/Oncol. 2011;33:185–9.
Marsh RA, Rao MB, Gefen A, Bellman D, Mehta PA, Khandelwal P, et al. Experience with alemtuzumab, fludarabine, and melphalan reduced-intensity conditioning hematopoietic cell transplantation in patients with nonmalignant diseases reveals good outcomes and that the risk of mixed chimerism depends on underlying disease, stem cell source, and alemtuzumab regimen. Biol Blood Marrow Transpl. 2015;21:1460–70.
Faraci M, Bagnasco F, Leoni M, Giardino S, Terranova P, Subissi L, et al. Evaluation of chimerism dynamics after allogeneic hematopoietic stem cell transplantation in children with nonmalignant diseases. Biol Blood Marrow Transpl. 2018;24:1088–93.
Kharfan-Dabaja MA, Kumar A, Ayala E, Aljurf M, Nishihori T, Marsh R, et al. Standardizing definitions of hematopoietic recovery, graft rejection, graft failure, poor graft function, and donor chimerism in allogeneic hematopoietic cell transplantation: a report on behalf of the american society for transplantation and cellular therapy. Transpl Cell Ther. 2021;27:642–9.
Karasu GT, Yesilipek MA, Karauzum SB, Uygun V, Manguoglu E, Kupesiz A, et al. The value of donor lymphocyte infusions in thalassemia patients at imminent risk of graft rejection following stem cell transplantation. Pediatr Blood Cancer. 2012;58:453–8.
Umeda K, Adachi S, Tanaka S, Miki M, Okada K, Hashii Y, et al. Comparison of second transplantation and donor lymphocyte infusion for donor mixed chimerism after allogeneic stem cell transplantation for nonmalignant diseases. Pediatr Blood Cancer. 2016;63:2221–9.
He M, Gui R, Zu Y, Li Z, Wang D, Mao Y, et al. Successful outcomes of second hematopoietic stem cell transplantation for graft failure in pediatric patients with severe aplastic anemia. Sci Rep. 2022;12:10528.
Cesaro S, De Latour RP, Tridello G, Pillon M, Carlson K, Fagioli F, et al. Second allogeneic stem cell transplant for aplastic anaemia: a retrospective study by the severe aplastic anaemia working party of the European society for blood and marrow transplantation. Br J Haematol. 2015;171:606–14.
Funding
This research was supported by the Basic Research and Cultivation Fund for Young Teachers of Zhengzhou University (grant number JC21854036).
Author information
Authors and Affiliations
Contributions
Jian Liu conceived and designed the study; Yumiao Mai analyzed the data, and wrote the draft; Zhaohe Jing, Linchao Zhao, Hongyun Niu, Simin Qiu, Pengpeng Dong collected the data. All authors have read and approved the final version of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
This study has been approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University (2024-KY-0934) and waived informed consent given the retrospective nature. The studies were conducted in accordance with the local legislation and institutional requirements, and all methods were performed in accordance with the relevant guidelines and regulations. All samples were collected as part of routine management/surveillance and were anonymized prior to research use.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, J., Mai, Y., Jing, Z. et al. Protective factors, management and prognosis of mixed chimerism after allogeneic hematopoietic stem cell transplantation for severe aplastic anemia in children. Bone Marrow Transplant 60, 811–819 (2025). https://doi.org/10.1038/s41409-025-02577-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41409-025-02577-3