Abstract
Background
PD-L1 promotes glycolysis in tumour cells. We observed a correlation between high PD-L1 expression and high 18F-FDG uptake in patients with pancreatic ductal adenocarcinoma (PDAC) in a previous study. This study aims to determine the usefulness of 18F-FDG PET/CT for evaluating the PD-L1 status in PDAC and to elucidate its rationality by integrated analyses.
Methods
For bioinformatics analysis, WGCNA, GSEA and TIMER were applied to analyse the pathways and hub genes associated with PD-L1 and glucose uptake. 18F-FDG uptake assay was used to determine the glucose uptake rate of PDAC cells in vitro. Related genes expression were verified by RT-PCR and western blot. A retrospective analysis was performed on 47 patients with PDAC who had undergone 18F-FDG PET/CT. Maximum standardised uptake values (SUVmax) were determined. The usefulness of SUVmax for evaluating PD-L1 status was determined by receiver operating characteristic (ROC) curve analysis.
Results
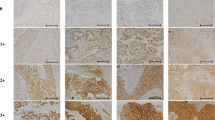
Bioinformatics analysis showed that several signalling pathways are associated with both PD-L1 expression and tumour glucose uptake, among which JAK-STAT may be an important one. By in vitro experiments, the regulatory role of PD-L1 on glucose uptake was demonstrated, and its dependency on the JAK-STAT pathway was also verified by the rescue study. The SUVmax of PD-L1-positive patients was significantly higher than PD-L1-negative in tumour cells (TCs) (6.1 ± 2.3 vs. 11.1 ± 4.2; P < 0.001), and in tumour-infiltrating immune cells (TIICs) (6.4 ± 3.2 vs. 8.4 ± 3.5; P < 0.001). In a multivariate analysis, SUVmax was significantly associated with PD-L1 expression in TCs and TIICs (P < 0.001 and P = 0.018, respectively). Using SUVmax cut-off values of 8.15 and 7.75, PD-L1 status in TCs and TIICs could be predicted with accuracies of 91.5% and 74.5%, respectively.
Conclusion
Higher 18F-FDG uptake by PDAC is associated with elevated PD-L1 expression. JAK-STAT is an important pathway that mediates PD-L1 to promote glucose uptake in PDAC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All analysed and derivative raw data are available on request.
References
Bear AS, Vonderheide RH, O’Hara MH. Challenges and opportunities for pancreatic cancer immunotherapy. Cancer Cell. 2020;38:788–802.
Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–49.
Reynolds EE, Doubeni CA, Sawhney MS, Kanjee Z. Should this patient be screened for pancreatic cancer?: grand rounds discussion from Beth Israel deaconess medical center. Ann Intern Med. 2020;173:914–21.
Zhang J, Dang F, Ren J, Wei W. Biochemical aspects of PD-L1 regulation in cancer immunotherapy. Trends Biochem Sci. 2018;43:1014–32.
O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, et al. Durvalumab with or without tremelimumab for patients with metastatic pancreatic ductal adenocarcinoma: a phase 2 randomized clinical trial. JAMA Oncol. 2019;5:1431–8.
Herrera FG, Ronet C, Ochoa de Olza M, Barras D, Crespo I, Andreatta M, et al. Low dose radiotherapy reverses tumor immune desertification and resistance to immunotherapy. Cancer Discov. 2021;12:108–33.
Shen X, Zhao B. Efficacy of PD-1 or PD-L1 inhibitors and PD-L1 expression status in cancer: meta-analysis. BMJ. 2018;362:k3529.
Li J, Yin L, Chen Y, An S, Xiong Y, Huang G, et al. PD-L1 correlated gene expression profiles and tumor infiltrating lymphocytes in pancreatic cancer. Int J Med Sci. 2021;18:3150–7.
Halbrook CJ, Lyssiotis CA. Employing metabolism to improve the diagnosis and treatment of pancreatic cancer. Cancer Cell. 2017;31:5–19.
Xia Q, Jia J, Hu C, Lu J, Li J, Xu H, et al. Tumor-associated macrophages promote PD-L1 expression in tumor cells by regulating PKM2 nuclear translocation in pancreatic ductal adenocarcinoma. Oncogene. 2021;41:865–77.
Chang C-H, Qiu J, O’Sullivan D, Buck Michael D, Noguchi T, Curtis Jonathan D, et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell. 2015;162:1229–41.
Chen R, Zhou X, Liu J, Huang G. Relationship between the expression of PD-1/PD-L1 and (18)F-FDG uptake in bladder cancer. Eur J Nucl Med Mol imaging. 2019;46:848–54.
Chen R, Chen Y, Huang G, Liu J. Relationship between PD-L1 expression and (18)F-FDG uptake in gastric cancer. Aging. 2019;11:12270–7.
Shi R, Bao X, Unger K, Sun J, Lu S, Manapov F, et al. Identification and validation of hypoxia-derived gene signatures to predict clinical outcomes and therapeutic responses in stage I lung adenocarcinoma patients. Theranostics. 2021;11:5061–76.
Xie C, Mao X, Huang J, Ding Y, Wu J, Dong S, et al. KOBAS 2.0: a web server for annotation and identification of enriched pathways and diseases. Nucleic Acids Res. 2011;39:W316–22.
Zeng Z, Cao Z, Zhang E, Huang H, Tang Y. Elevated CDK5R1 predicts worse prognosis in hepatocellular carcinoma based on TCGA data. Biosci Rep. 2021;41:BSR20203594.
Lucignani G, Paganelli G, Bombardieri E. The use of standardized uptake values for assessing FDG uptake with PET in oncology: a clinical perspective. Nucl Med Commun. 2004;25:651–6.
Berghmans T, Dusart M, Paesmans M, Hossein-Foucher C, Buvat I, Castaigne C, et al. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12.
Kinahan PE, Fletcher JW. Positron emission tomography-computed tomography standardized uptake values in clinical practice and assessing response to therapy. Semin Ultrasound CT MR. 2010;31:496–505.
Zhang S, Zhang R, Gong W, Wang C, Zeng C, Zhai Y, et al. Positron emission tomography-computed tomography parameters predict efficacy of immunotherapy in head and neck squamous cell carcinomas. Front Oncol. 2021;11:728040.
Cha JH, Chan LC, Li CW, Hsu JL, Hung MC. Mechanisms controlling PD-L1 expression in cancer. Mol Cell. 2019;76:359–70.
Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48:434–52.
Yu J, Qin B, Moyer AM, Nowsheen S, Tu X, Dong H, et al. Regulation of sister chromatid cohesion by nuclear PD-L1. Cell Res. 2020;30:590–601.
Gao Y, Nihira NT, Bu X, Chu C, Zhang J, Kolodziejczyk A, et al. Acetylation-dependent regulation of PD-L1 nuclear translocation dictates the efficacy of anti-PD-1 immunotherapy. Nat Cell Biol. 2020;22:1064–75.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74.
Qin C, Yang G, Yang J, Ren B, Wang H, Chen G, et al. Metabolism of pancreatic cancer: paving the way to better anticancer strategies. Mol Cancer. 2020;19:50.
Wang S, Li J, Xie J, Liu F, Duan Y, Wu Y, et al. Programmed death ligand 1 promotes lymph node metastasis and glucose metabolism in cervical cancer by activating integrin beta4/SNAI1/SIRT3 signaling pathway. Oncogene. 2018;37:4164–80.
Cao D, Qi Z, Pang Y, Li H, Xie H, Wu J, et al. Retinoic acid-related orphan receptor C regulates proliferation, glycolysis, and chemoresistance via the PD-L1/ITGB6/STAT3 signaling axis in bladder cancer. Cancer Res. 2019;79:2604–18.
Ma P, Xing M, Han L, Gan S, Ma J, Wu F, et al. High PDL1 expression drives glycolysis via an Akt/mTOR/HIF1alpha axis in acute myeloid leukemia. Oncol Rep. 2020;43:999–1009.
Zhao Y, Ma T, Zou D. Identification of unique transcriptomic signatures and hub genes through RNA sequencing and integrated WGCNA and PPI network analysis in nonerosive reflux disease. J Inflamm Res. 2021;14:6143–56.
Brooks AJ, Putoczki T. JAK-STAT signalling pathway in cancer. Cancers. 2020;12:1971.
Valle-Mendiola A, Soto-Cruz I. Energy metabolism in cancer: the roles of STAT3 and STAT5 in the regulation of metabolism-related genes. Cancers. 2020;12:124.
Dodington DW, Desai HR, Woo M. JAK/STAT—emerging players in metabolism. Trends Endocrinol Metab: TEM. 2018;29:55–65.
Acknowledgements
We are grateful to the physicians, nurses, and all the patients who participated in this research.
Funding
The study was sponsored by the National Key Research and Development Program of China (2021YFA0910000 and 2020YFA0909000), the Interdisciplinary Program of Shanghai Jiao Tong University (ZH2018QNA41), and the National Natural Science Foundation of China (Nos. 81771858 and 82171972).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study’s conception and design. Material preparation, data collection and analysis were performed by Jiajin Li, XiaQing, Cheng Wang, Liangrong Wan and Haiqin Bao. The first draft of the manuscript was written by Jiajin Li, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were approved by the Institutional Review Board of Shanghai Jiao Tong University-affiliated Ren Ji Hospital and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. This article does not describe any studies with animals performed by any of the authors.
Consent for publication
Written informed consent was obtained from the patient for publication of this manuscript and any accompanying images.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Li, J., Chen, R., Chen, Y. et al. Relationship between the expression of PD-L1 and 18F-FDG uptake in pancreatic ductal adenocarcinoma. Br J Cancer 129, 541–550 (2023). https://doi.org/10.1038/s41416-023-02297-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-023-02297-9
This article is cited by
-
PD-L1 imaging with [99mTc]NM-01 SPECT/CT is associated with metabolic response to pembrolizumab with/without chemotherapy in advanced lung cancer
British Journal of Cancer (2025)
-
Predicting programmed death-ligand 1 (PD-L1) expression with fluorine-18 fluorodeoxyglucose ([18F]FDG) positron emission tomography/computed tomography (PET/CT) metabolic parameters in resectable non-small cell lung cancer
European Radiology (2024)