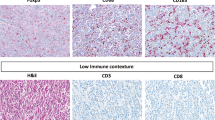
Abstract
Background
Pancreatic cancer (PC) is characterized by abnormally fibrotic mesenchyme, which notably influences on the effectiveness of immunotherapy. Low-dose arsenic trioxide (ATO, 1.0 μM) can inhibit the activation of pancreatic stellate cells (PSCs) and affect fibrosis, which is a potential strategy for enhancing the sensitivity to immunotherapy.
Methods
Extracellular matrix (ECM) models were employed to assess the regulatory effects of ATO on ECM and peripheral blood mononuclear cells. Orthotopic C57BL/6J models were utilized to evaluate the influence of ATO on CD8+T cell infiltration and immunotherapy in PC. Additionally, nanomaterials loaded with ATO designed to specifically target PSCs (scAbFAP-α-HMSNs-PAA-ATO) were produced to enhance targeting effects of ATO.
Results
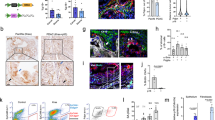
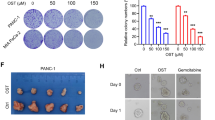
Low-dose ATO (1.0 μM) suppressed PSCs activation, exhibiting potential for synergistic immunotherapy. Under low-dose ATO intervention, ECM underwent remodeling, leading to increases in CD8+T cell infiltration, thereby enhancing anti-PD-L1 therapy effect. We further demonstrated that low-dose ATO remodeled ECM by regulating the expression of LOXL3 in PSCs. scAbFAP-α-HMSNs-PAA-ATO exhibited improved targeting capabilities, and enhanced capacity to inhibit fibrosis and sensitize immunotherapy.
Conclusions
Our research reveals that low-dose ATO, by regulating LOXL3, remodels the ECM and enhances CD8+T cell infiltration, thus sensitizing the efficacy of immunotherapy, which provides a novel strategy for comprehensive treatment to PC.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Mortezaee K. Enriched cancer stem cells, dense stroma, and cold immunity: Interrelated events in pancreatic cancer. J Biochem Mol Toxicol. 2021;35:e22708.
Henriksen A, Dyhl-Polk A, Chen I, Nielsen D. Checkpoint inhibitors in pancreatic cancer. Cancer Treat Rev. 2019;78:17–30.
Majidpoor J, Mortezaee K. The efficacy of PD-1/PD-L1 blockade in cold cancers and future perspectives. Clin Immunol. 2021;226:108707.
Nilsson M, Adamo H, Bergh A, & Halin Bergström S. Inhibition of lysyl oxidase and lysyl oxidase-like enzymes has tumour-promoting and tumour-suppressing roles in experimental prostate cancer. Sci Rep. 2016;6:19608.
Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet. 2018;391:2128–39.
Doi T, Muro K, Ishii H, Kato T, Tsushima T, Takenoyama M, et al. A phase I study of the anti-CC chemokine receptor 4 antibody, mogamulizumab, in combination with nivolumab in patients with advanced or metastatic solid tumors. Clin Cancer Res. 2019;25:6614–22.
Hellmann MD, Kim TW, Lee CB, Goh BC, Miller WH, Oh DY, et al. Phase Ib study of atezolizumab combined with cobimetinib in patients with solid tumors. Ann Oncol. 2019;30:1134–42.
Soignet SL, Maslak P, Wang Z-G, Jhanwar S, Calleja E, Dardashti LJ, et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N Engl J Med. 1998;339:1341–8.
Qian C, Wang Y, Chen Y, Zeng L, Zhang Q, Shuai X, et al. Suppression of pancreatic tumor growth by targeted arsenic delivery with anti-CD44v6 single chain antibody conjugated nanoparticles. Biomaterials. 2013;34:6175–84.
Chen J, Jin Z, Zhang S, Zhang X, Li P, Yang H, et al. Arsenic trioxide elicits prophylactic and therapeutic immune responses against solid tumors by inducing necroptosis and ferroptosis. Cell Mol Immunol. 2023;20:51–64.
Chen Z, Zhang H, Yang L, Jiang H, Guo S, Li Y, et al. Construction of a metabolomics profile of arsenic trioxide effect in gastric carcinoma cell line SGC7901. Acta Biochim Biophys Sin. 2016;48:474–81.
Duan X, Li T, Han X, Ren J, Chen P, Li H, et al. The antitumor effect of arsenic trioxide on hepatocellular carcinoma is enhanced by andrographolide. Oncotarget. 2017;8:90905–15.
Gao J, Wang G, Wu J, Zuo Y, Zhang J, Chen J. Arsenic trioxide inhibits Skp2 expression to increase chemosensitivity to gemcitabine in pancreatic cancer cells. Am J Transl Res. 2019;11:991–7.
Wang QQ, Jiang Y, Naranmandura H. Therapeutic strategy of arsenic trioxide in the fight against cancers and other diseases. Metallomics. 2020;12:326–36.
Luo F, Zhuang Y, Sides MD, Sanchez CG, Shan B, White ES, et al. Arsenic trioxide inhibits transforming growth factor-β1-induced fibroblast to myofibroblast differentiation in vitro and bleomycin induced lung fibrosis in vivo. Respir Res. 2014;15:51.
Zhao Y, Yao H, Yang K, Han S, Chen S, Li Y, et al. Arsenic trioxide-loaded nanoparticles enhance the chemosensitivity of gemcitabine in pancreatic cancer via the reversal of pancreatic stellate cell desmoplasia by targeting the AP4/galectin-1 pathway. Biomater Sci. 2022;10:5989–6002.
Pothula SP, Pirola RC, Wilson JS, Apte MV. Pancreatic stellate cells: aiding and abetting pancreatic cancer progression. Pancreatology. 2020;20:409–18.
Laurentino TS, Soares RDS, Marie SK N & Oba-Shinjo SM. LOXL3 function beyond amino oxidase and role in pathologies, including cancer. Int J Mol Sci. 2019;20:3587.
Wang, N., Zhou, X., Tang, F., Wang, X. & Zhu, X. Identification of LOXL3-associating immune infiltration landscape and prognostic value in hepatocellular carcinoma. Virchows Archiv Int J Pathol https://doi.org/10.1007/s00428-021-03193-4 (2021).
Santamaría PG, Floristán A, Fontanals-Cirera B, Vázquez-Naharro A, Santos V, Morales S, et al. Lysyl oxidase-like 3 is required for melanoma cell survival by maintaining genomic stability. Cell Death Differ. 2018;25:935–50.
Weniger M, Honselmann KC, & Liss AS. The extracellular matrix and pancreatic cancer: a complex relationship. Cancers. 2018;10:316.
DuFort CC, DelGiorno KE, Hingorani SR. Mounting pressure in the microenvironment: fluids, solids, and cells in pancreatic ductal adenocarcinoma. Gastroenterology. 2016;150:1545–1557.e1542.
Francescone R, Barbosa Vendramini-Costa D, Franco-Barraza J, Wagner J, Muir A, Lau AN, et al. Netrin G1 promotes pancreatic tumorigenesis through cancer-associated fibroblast-driven nutritional support and immunosuppression. Cancer Discov. 2021;11:446–79.
Wu D, Zhu ZQ, Tang HX, Shi ZE, Kang J, Liu Q, et al. Efficacy-shaping nanomedicine by loading calcium peroxide into tumor microenvironment-responsive nanoparticles for the antitumor therapy of prostate cancer. Theranostics. 2020;10:9808–29.
Shao Y, Wang L, Fu J, Shi C, Xu J, Zhu Y. Efficient free radical generation against cancer cells by low-dose X-ray irradiation with a functional SPC delivery nanosystem. J Mater Chem B. 2016;4:5863–72.
Patsouras D, Papaxoinis K, Kostakis A, Safioleas MC, Lazaris AC, Nicolopoulou-Stamati P. Fibroblast activation protein and its prognostic significance in correlation with vascular endothelial growth factor in pancreatic adenocarcinoma. Mol Med Rep. 2015;11:4585–90.
Yamamoto K, Iwadate D, Kato H, Nakai Y, Tateishi K, Fujishiro M. Targeting the metabolic rewiring in pancreatic cancer and its tumor microenvironment. Cancers. 2022;14:4351.
Kalim KW, Yang JQ, Wunderlich M, Modur V, Nguyen P, Li Y, et al. Targeting of Cdc42 GTPase in regulatory T cells unleashes antitumor T-cell immunity. J Immunother Cancer. 2022;10:e004806.
LaRue MM, Parker S, Puccini J, Cammer M, Kimmelman AC, & Bar-Sagi D. Metabolic reprogramming of tumor-associated macrophages by collagen turnover promotes fibrosis in pancreatic cancer. Proc Natl Acad Sci. 2022;119:e2119168119.
Peng DH, Rodriguez BL, Diao L, Chen L, Wang J, Byers LA, et al. Collagen promotes anti-PD-1/PD-L1 resistance in cancer through LAIR1-dependent CD8(+) T cell exhaustion. Nat Commun. 2020;11:4520.
Garcia Garcia CJ, Huang Y, Fuentes NR, Turner MC, Monberg ME, Lin D, et al. Stromal HIF2 regulates immune suppression in the pancreatic cancer microenvironment. Gastroenterology. 2022;162:2018–31.
Falcomatà C, Bärthel S, Widholz SA, Schneeweis C, Montero JJ, Toska A, et al. Selective multi-kinase inhibition sensitizes mesenchymal pancreatic cancer to immune checkpoint blockade by remodeling the tumor microenvironment. Nat Cancer. 2022;3:318–36.
Ro S-H, Bae J, Jang Y, Myers JF, Chung S, Yu J, et al. Arsenic toxicity on metabolism and autophagy in adipose and muscle tissues. Antioxidants. 2022;11:689.
Khairul I, Wang QQ, Jiang YH, Wang C, Naranmandura H. Metabolism, toxicity and anticancer activities of arsenic compounds. Oncotarget. 2017;8:23905–26.
Tian Z, Tan Y, Lin X, Su M, Pan L, Lin L, et al. Arsenic trioxide sensitizes pancreatic cancer cells to gemcitabine through downregulation of the TIMP1/PI3K/AKT/mTOR axis. Transl Res J Lab Clin Med. 2023;255:66–76.
Zhang J, Li C, Zheng Y, Lin Z, Zhang Y, Zhang Z. Inhibition of angiogenesis by arsenic trioxide via TSP-1-TGF-β1-CTGF-VEGF functional module in rheumatoid arthritis. Oncotarget. 2017;8:73529–46.
Hosein AN, Brekken RA, Maitra A. Pancreatic cancer stroma: an update on therapeutic targeting strategies. Nat Rev Gastroenterol Hepatol. 2020;17:487–505.
Najafi M, Mortezaee K, Majidpoor J. Stromal reprogramming: a target for tumor therapy. Life Sci. 2019;239:117049.
Farhood B, Najafi M, Mortezaee K. Cancer-associated fibroblasts: secretions, interactions, and therapy. J Cell Biochem. 2019;120:2791–2800.
Fejza A, Polano M, Camicia L, Poletto E, Carobolante G, Toffoli G, et al. The efficacy of Anti-PD-L1 treatment in melanoma is associated with the expression of the ECM molecule EMILIN2. Int J Mol Sci. 2021;22:7511.
Kuczek DE, Larsen AMH, Thorseth M-L, Carretta M, Kalvisa A, Siersbæk MS, et al. Collagen density regulates the activity of tumor-infiltrating T cells. J ImmunoTher Cancer 2019;7:68.
Jiang C, Wang M, Yao W, Lv G, Liu X, & Wang G. Comprehensive analysis on prognosis and immune infiltration of lysyl oxidase family members in pancreatic adenocarcinoma with experimental verification. Front Mol Biosci. 2022;9:778857.
Ferrara B, Pignatelli C, Cossutta M, Citro A, Courty J, & Piemonti L. The extracellular matrix in pancreatic cancer: description of a complex network and promising therapeutic options. Cancers. 2021;13:4442.
Ma L, Huang C, Wang X-J, Xin DE, Wang L-S, Zou QC, et al. Lysyl Oxidase 3 is a dual-specificity enzyme involved in STAT3 deacetylation and deacetylimination modulation. Mol Cell. 2017;65:296–309.
Tang F, Li L, Chen D. Mesoporous silica nanoparticles: synthesis, biocompatibility and drug delivery. Adv Mater. 2012;24:1504–34.
Kawase T, Yasui Y, Nishina S, Hara Y, Yanatori I, Tomiyama Y, et al. Fibroblast activation protein-α-expressing fibroblasts promote the progression of pancreatic ductal adenocarcinoma. BMC Gastroenterol. 2015;15:109.
Funding
This study was jointly supported by the National Natural Science Foundation of China (82203036, 81874057, 82373025, 82003175), Guangzhou Science and Technology Plan Project (SL2024A04J01991), China Postdoctoral Science Foundation (2023M734051), Medical Scientific Research Foundation of Guangdong Province of China (A2016210), Science and Technology Program of Guangzhou, China (202102020161), Guangdong Basic and Applied Basic Research Foundation (2024A1515012799, 2021A1515110240).
Author information
Authors and Affiliations
Contributions
YZ, YL, and JZ contributed equally to this work. YH, GL, KH, and JL developed the concept and supervised the experiments. YH wrote the original draft. YH, JL, GL, KH, YZ, YL, and JZ wrote the manuscript. YZ, YL, JZ, ZZ, TG, and SC carried out the experiments and performed the statistical analyses. YZ and YL performed the data analysis. YL, ZZ, and TG provided the materials.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval and consent to participate
The use of human samples was approved by Sun Yat-sen Memorial Hospital Ethics Committee and the informed consent from all participants were obtained (SYSKY-2023-116-01). All methods involved in this study were carried out in accordance with relevant guidelines and regulations. All animals care and experimental procedures were conducted according to the National Research Council’s Guidelines for the Care and Use of Laboratory Animals, and were approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University Cancer Center (L102012022040E).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhao, Y., Li, Y., Zou, J. et al. Low-dose arsenic trioxide inhibits pancreatic stellate cell activation via LOXL3 expression to enhance immunotherapy in pancreatic cancer. Br J Cancer 131, 1928–1941 (2024). https://doi.org/10.1038/s41416-024-02880-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02880-8