Abstract
Background
Although phytoestrogens modulated pancreatic tumour growth in experimental studies, it remains unclear whether phytoestrogen intake is associated with pancreatic cancer.
Methods
Of 92,278 persons who completed the Diet History Questionnaire in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial, 346 were diagnosed with pancreatic cancer within a median follow-up of 9.4 years. Cox proportional hazards regression was used to evaluate pancreatic cancer risk in relation to phytoestrogen intake.
Results
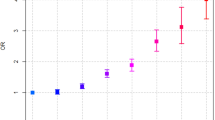
After adjustment for confounders, intakes of glycitein and formononetin were associated with a reduced risk of pancreatic cancer [highest vs. lowest quartile, hazard ratio (HR) (95% confidence interval (CI)) for glycitein: 0.60 (0.39, 0.92); P for linear trend: 0.01; HR for formononetin: 0.51 (0.37, 0.70); P for linear trend: 0.005]. These associations were stronger and their linear trends across the quartiles of intakes were more statistically significant among ever smokers than never-smokers. A reduced risk was also observed for ever smokers in the highest quartile of total isoflavones or daidzein compared with those in the lowest quartile.
Conclusions
Our study suggests that high intakes of total isoflavones and some individual isoflavones were inversely associated with pancreatic cancer risk, but this potential protective effect was confined to ever smokers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
Data availability
PLCO data analysed in the present study will be provided upon request, pending approval from the National Cancer Institute. Data requests can be made via the Cancer Data Access System at https://cdas.cancer.gov/plco/.
References
Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33.
NIH National Cancer Institute SEER Program. Cancer Stat Facts: Pancreatic Cancer. 2024. https://seer.cancer.gov/statfacts/html/pancreas.html.
Klein AP. Pancreatic cancer epidemiology: understanding the role of lifestyle and inherited risk factors. Nat Rev Gastroenterol Hepatol. 2021;18:493–502.
Torrens-Mas M, Roca P. Phytoestrogens for cancer prevention and treatment. Biology. 2020;9:427.
Murphy PA, Barua K, Hauck CC. Solvent extraction selection in the determination of isoflavones in soy foods. J Chromatogr B Anal Technol Biomed Life Sci. 2002;777:129–38.
Rietjens I, Louisse J, Beekmann K. The potential health effects of dietary phytoestrogens. Br J Pharm. 2017;174:1263–80.
Andres S, Abraham K, Appel KE, Lampen A. Risks and benefits of dietary isoflavones for cancer. Crit Rev Toxicol. 2011;41:463–506.
Yamagiwa Y, Sawada N, Shimazu T, Yamaji T, Goto A, Takachi R, et al. Soy food intake and pancreatic cancer risk: the Japan Public Health Center-based prospective study. Cancer Epidemiol Biomark Prev. 2020;29:1214–21.
McGuinness EE, Morgan RG, Wormsley KG. Fate of pancreatic nodules induced by raw soya flour in rats. Gut. 1987;28:207–12.
Lyn-Cook BD, Stottman HL, Yan Y, Blann E, Kadlubar FF, Hammons GJ. The effects of phytoestrogens on human pancreatic tumor cells in vitro. Cancer Lett. 1999;142:111–9.
Bhardwaj V, Tadinada SM, Jain A, Sehdev V, Daniels CK, Lai JC, et al. Biochanin A reduces pancreatic cancer survival and progression. Anticancer Drugs. 2014;25:296–302.
Zhu CS, Pinsky PF, Kramer BS, Prorok PC, Purdue MP, Berg CD, et al. The prostate, lung, colorectal, and ovarian cancer screening trial and its associated research resource. J Natl Cancer Inst. 2013;105:1684–93.
Subar AF, Thompson FE, Kipnis V, Midthune D, Hurwitz P, McNutt S, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires: the Eating at America’s Table Study. Am J Epidemiol. 2001;154:1089–99.
Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–26.
NIH National Cancer Institute Cancer Data Access System. Main Questionnaires. 2024. https://cdas.cancer.gov/learn/plco/early-qx/dhq/.
Bhagwat S, Haytowithz DB, Holden JM. USDA Database for the Isoflavone Content of Selected Foods. Release 2.0. September 2008. 2024. https://www.ars.usda.gov/ARSUserFiles/80400525/data/isoflav/isoflav_r2.pdf.
Cancer Data Access System. DHQ dataset: data dictionary, appendix 2: nutrient. U.S. Department of Health and Human Services. 2024. https://cdas.cancer.gov/datasets/plco/98/.
Capasso M, Franceschi M, Rodriguez-Castro KI, Crafa P, Cambie G, Miraglia C, et al. Epidemiology and risk factors of pancreatic cancer. Acta Biomed. 2018;89:141–6.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57.
Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S.
Fan Y, Wang M, Li Z, Jiang H, Shi J, Shi X, et al. Intake of soy, soy isoflavones and soy protein and risk of cancer incidence and mortality. Front Nutr. 2022;9:847421.
Messina M, Nagata C, Wu AH. Estimated Asian adult soy protein and isoflavone intakes. Nutr Cancer. 2006;55:1–12.
Wang Q, Huang H, Zhao N, Ni X, Udelsman R, Zhang Y. Phytoestrogens and thyroid cancer risk: a population-based case-control study in Connecticut. Cancer Epidemiol Biomark Prev. 2020;29:500–8.
Rizzo G, Baroni L. Soy, soy foods and their role in vegetarian diets. Nutrients. 2018;10:43.
Tamang JP, Cotter PD, Endo A, Han NS, Kort R, Liu SQ, et al. Fermented foods in a global age: east meets west. Compr Rev Food Sci Food Saf. 2020;19:184–217.
Guo JM, Xiao BX, Dai DJ, Liu Q, Ma HH. Effects of daidzein on estrogen-receptor-positive and negative pancreatic cancer cells in vitro. World J Gastroenterol. 2004;10:860–3.
Gundogdu G, Dodurga Y, Cetin M, Secme M, Cicek B. The cytotoxic and genotoxic effects of daidzein on MIA PaCa-2 human pancreatic carcinoma cells and HT-29 human colon cancer cells. Drug Chem Toxicol. 2020;43:581–7.
Sotoca AM, Ratman D, van der Saag P, Strom A, Gustafsson JA, Vervoort J, et al. Phytoestrogen-mediated inhibition of proliferation of the human T47D breast cancer cells depends on the ERalpha/ERbeta ratio. J Steroid Biochem Mol Biol. 2008;112:171–8.
Pons DG, Nadal-Serrano M, Torrens-Mas M, Oliver J, Roca P. The phytoestrogen genistein affects breast cancer cells treatment depending on the ERalpha/ERbeta ratio. J Cell Biochem. 2016;117:218–29.
Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–5.
Konduri S, Schwarz RE. Estrogen receptor beta/alpha ratio predicts response of pancreatic cancer cells to estrogens and phytoestrogens. J Surg Res. 2007;140:55–66.
Ong SKL, Shanmugam MK, Fan L, Fraser SE, Arfuso F, Ahn KS, et al. Focus on formononetin: anticancer potential and molecular targets. Cancers. 2019;11:611.
Tay KC, Tan LT, Chan CK, Hong SL, Chan KG, Yap WH, et al. Formononetin: a review of its anticancer potentials and mechanisms. Front Pharm. 2019;10:820.
Wang Q, Ru M, Zhang Y, Kurbanova T, Boffetta P. Dietary phytoestrogen intake and lung cancer risk: an analysis of the Prostate, Lung, Colorectal and Ovarian (PLCO) cancer screening trial. Carcinogenesis. 2021;42:1250–9.
Nothlings U, Wilkens LR, Murphy SP, Hankin JH, Henderson BE, Kolonel LN. Vegetable intake and pancreatic cancer risk: the multiethnic cohort study. Am J Epidemiol. 2007;165:138–47.
Woo HD, Kim J. Dietary flavonoid intake and smoking-related cancer risk: a meta-analysis. PLoS One. 2013;8:e75604.
Cui Y, Morgenstern H, Greenland S, Tashkin DP, Mao JT, Cai L, et al. Dietary flavonoid intake and lung cancer-a population-based case-control study. Cancer. 2008;112:2241–8.
Speisky H, Shahidi F, Costa de Camargo A, Fuentes J. Revisiting the oxidation of flavonoids: loss, conservation or enhancement of their antioxidant properties. Antioxidants. 2022;11:133.
Bravo L. Polyphenols: chemistry, dietary sources, metabolism, and nutritional significance. Nutr Rev. 1998;56:317–33.
Buiatti E, Munoz N, Kato I, Vivas J, Muggli R, Plummer M, et al. Determinants of plasma anti-oxidant vitamin levels in a population at high risk for stomach cancer. Int J Cancer. 1996;65:317–22.
Fu YS, Kang N, Yu Y, Mi Y, Guo J, Wu J, et al. Polyphenols, flavonoids and inflammasomes: the role of cigarette smoke in COPD. Eur Respir Rev. 2022;31:220028.
Greten FR, Grivennikov SI. Inflammation and cancer: triggers, mechanisms, and consequences. Immunity. 2019;51:27–41.
Kojima K, Asai K, Kubo H, Sugitani A, Kyomoto Y, Okamoto A, et al. Isoflavone aglycones attenuate cigarette smoke-induced emphysema via suppression of neutrophilic inflammation in a COPD murine model. Nutrients. 2019;11:2023.
Steiner C, Peters WH, Gallagher EP, Magee P, Rowland I, Pool-Zobel BL. Genistein protects human mammary epithelial cells from benzo(a)pyrene-7,8-dihydrodiol-9,10-epoxide and 4-hydroxy-2-nonenal genotoxicity by modulating the glutathione/glutathione S-transferase system. Carcinogenesis. 2007;28:738–48.
Khan TH, Prasad L, Anuradha, Sultana S. Soy isoflavones inhibits the genotoxicity of benzo(a)pyrene in Swiss albino mice. Hum Exp Toxicol. 2005;24:149–55.
Lee YS, Seo JS, Chung HT, Jang JJ. Inhibitory effects of biochanin A on mouse lung tumor induced by benzo(a)pyrene. J Korean Med Sci. 1991;6:325–8.
Lee YS, Kim TH, Cho KJ, Jang JJ. Inhibitory effects of biochanin A on benzo(a)pyrene induced carcinogenesis in mice. Vivo. 1992;6:283–6.
Delgado ME, Haza AI, Arranz N, Garcia A, Morales P. Dietary polyphenols protect against N-nitrosamines and benzo(a)pyrene-induced DNA damage (strand breaks and oxidized purines/pyrimidines) in HepG2 human hepatoma cells. Eur J Nutr. 2008;47:479–90.
Schwarz D, Kisselev P, Roots I. CYP1A1 genotype-selective inhibition of benzo [a]pyrene activation by quercetin. Eur J Cancer. 2005;41:151–8.
Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol Vitr. 2006;20:187–210.
Rowland I, Faughnan M, Hoey L, Wahala K, Williamson G, Cassidy A. Bioavailability of phyto-oestrogens. Br J Nutr. 2003;89:S45–58.
Micek A, Godos J, Brzostek T, Gniadek A, Favari C, Mena P, et al. Dietary phytoestrogens and biomarkers of their intake in relation to cancer survival and recurrence: a comprehensive systematic review with meta-analysis. Nutr Rev. 2021;79:42–65.
French MR, Thompson LU, Hawker GA. Validation of a phytoestrogen food frequency questionnaire with urinary concentrations of isoflavones and lignan metabolites in premenopausal women. J Am Coll Nutr. 2007;26:76–82.
Jaceldo-Siegl K, Fraser GE, Chan J, Franke A, Sabate J. Validation of soy protein estimates from a food-frequency questionnaire with repeated 24-h recalls and isoflavonoid excretion in overnight urine in a western population with a wide range of soy intakes. Am J Clin Nutr. 2008;87:1422–7.
van der Schouw YT, Kreijkamp-Kaspers S, Peeters PH, Keinan-Boker L, Rimm EB, Grobbee DE. Prospective study on usual dietary phytoestrogen intake and cardiovascular disease risk in western women. Circulation. 2005;111:465–71.
Schabath MB, Hernandez LM, Wu X, Pillow PC, Spitz MR. Dietary phytoestrogens and lung cancer risk. JAMA. 2005;294:1493–504.
Cotterchio M, Boucher BA, Manno M, Gallinger S, Okey A, Harper P. Dietary phytoestrogen intake is associated with reduced colorectal cancer risk. J Nutr. 2006;136:3046–53.
Reger MK, Zollinger TW, Liu Z, Jones J, Zhang J. Urinary phytoestrogens and cancer, cardiovascular, and all-cause mortality in the continuous National Health and Nutrition Examination Survey. Eur J Nutr. 2016;55:1029–40.
Filiberto AC, Mumford SL, Pollack AZ, Zhang C, Yeung EH, Perkins NJ, et al. Habitual dietary isoflavone intake is associated with decreased C-reactive protein concentrations among healthy premenopausal women. J Nutr. 2013;143:900–6.
Reger MK, Zollinger TW, Liu Z, Jones J, Zhang J. Association between urinary phytoestrogens and C-reactive protein in the continuous National Health and Nutrition Examination Survey. J Am Coll Nutr. 2017;36:434–41.
Gukovsky I, Li N, Todoric J, Gukovskaya A, Karin M. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology. 2013;144:1199–209 e1194.
Acknowledgements
We are grateful to the National Cancer Institute for providing access to the data from the PLCO Cancer Screening Trial. The statements contained herein are solely those of the authors and do not represent or imply concurrence or endorsement by the National Cancer Institute.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows—C.L., M.R., H.F., J.W. and J.Z.: participated in the review and revision of the manuscript; C.L. and J.Z.: designed and conducted research; C.L.: analysed data; C.L. and J.Z.: wrote the paper; J.Z.: had primary responsibility for final content; All authors: read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics approval
All methods were conducted in compliance with the relevant ethical guidelines and regulations. The PLCO Cancer Screening Trial was approved by both the institutional review boards of the National Cancer Institute and all participating screening centres
Informed consent
Written informed consent was obtained from all participants.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liu, C., Reger, M., Fan, H. et al. Dietary intake of isoflavones and coumestrol and risk of pancreatic cancer in the prostate, lung, colorectal, and ovarian cancer screening trial. Br J Cancer 132, 266–275 (2025). https://doi.org/10.1038/s41416-024-02929-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41416-024-02929-8