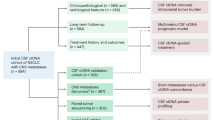
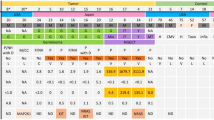
Abstract
Background
Cystic brain metastases (CBM) present significant clinical challenges due to their heterogeneity and the limitations of current diagnostic methods in guiding treatment. Traditional tissue biopsies are invasive and may not capture tumour heterogeneity, while plasma circulating tumour DNA (ctDNA) analysis is impeded by the blood-brain barrier, leading to low sensitivity for detecting intracranial lesions. These limitations create a critical gap in the personalised management of patients with CBM.
Methods
We evaluated the utility of cyst fluid ctDNA as a minimally invasive biomarker for genetic profiling and treatment monitoring in CBM patients. ctDNA was extracted from cyst fluid, tumour tissue, plasma, and cerebrospinal fluid (CSF) samples collected from 18 patients. NGS was performed to analyse genetic mutations. Mutation detection rates and genetic heterogeneity were compared across different sample types. Dynamic changes in ctDNA mutation abundance in cyst fluid were assessed in relation to treatment responses.
Results
Cyst fluid ctDNA demonstrated a higher mutation detection rate and captured more significant genetic heterogeneity than plasma ctDNA and, in some cases, even matched tissue samples. Clinically significant mutations, including actionable driver genes such as EGFR and TP53, were identified in cyst fluid ctDNA but were undetectable in plasma. Moreover, dynamic changes in the abundance of ctDNA mutations in cyst fluid correlated with treatment responses, indicating its potential for real-time therapeutic efficacy monitoring.
Conclusions
Cyst fluid ctDNA provides a sensitive and comprehensive method for capturing the genetic landscape of CBM, effectively overcoming the limitations of tissue biopsies and plasma ctDNA analysis. By establishing a real-time molecular surveillance network, cyst fluid ctDNA analysis redefines precision neuro-oncology paradigms, transitioning CBM management from static histomolecular snapshots to adaptive therapeutic ecosystems.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 24 print issues and online access
269,00 € per year
only 11,21 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Data availability
The datasets generated during and/or analysed during the current study are not publicly available due to being part of an ongoing long-term study but are available from the corresponding author on reasonable request.
References
Nayak L, Lee EQ, Wen PY. Epidemiology of brain metastases. Curr Oncol Rep. 2012;14:48–54.
Rehman AU, Khan P, Maurya SK, Siddiqui JA, Santamaria-Barria JA, Batra SK, et al. Liquid biopsies to occult brain metastasis. Mol Cancer. 2022;21:113.
Aizer AA, Lamba N, Ahluwalia MS, Aldape K, Boire A, Brastianos PK, et al. Brain metastases: a Society for Neuro-Oncology (SNO) consensus review on current management and future directions. Neuro Oncol. 2022;24:1613–46.
Xu YB, Zhang Y, Song Z, Wang W, Shao L. Treatment and prognosis of solid and cystic brain metastases in patients with non-small-cell lung cancer. Cancer Manag Res. 2021;13:6309–17.
Brigell RH, Cagney DN, Martin AM, Besse LA, Catalano PJ, Lee EQ, et al. Local control after brain-directed radiation in patients with cystic versus solid brain metastases. J Neurooncol. 2019;142:355–63.
Lin X, DeAngelis LM. Treatment of brain metastases. J Clin Oncol. 2015;33:3475–84.
Tabouret E, Chinot O, Metellus P, Tallet A, Viens P, Gonçalves A. Recent trends in epidemiology of brain metastases: an overview. Anticancer Res. 2012;32:4655–62.
Boire A, Brastianos PK, Garzia L, Valiente M. Brain metastasis. Nat Rev Cancer. 2020;20:4–11.
Barnholtz-Sloan JS, Sloan AE, Davis FG, Vigneau FD, Lai P, Sawaya RE. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22:2865–72.
Soffietti R, Rudà R, Mutani R. Management of brain metastases. J Neurol. 2002;249:1357–69.
Amidon RF, Livingston K, Kleefisch CJ, Martens M, Straza M, Puckett L, et al. Cystic brain metastasis outcomes after gamma knife radiation therapy. Adv Radiat Oncol. 2024;9:101304.
Patel KR, Prabhu RS, Kandula S, Murphy ES. Cystic brain metastases: an evidence-based review of management. Clin Neurol Neurosurg. 2014;125:30–34.
Halasz LM, Rockhill JK. Stereotactic radiosurgery for the management of brain metastases. Neurosurg Clin North Am. 2013;24:521–30.
Quigley MR, Fukui O. The impact of cystic metastases on the neurocognition of patients with brain metastases. J Neuro-Oncol. 2010;98:107–15.
Fidler IJ, Balasubramanian K, Lin Q, Kim SW. The brain microenvironment and cancer metastasis. Mol Oncol. 2020;14:2531–48.
Li YS, Jiang BY, Yang JJ, Zhang XC, Zhong WZ. Unique genetic profiles from cerebrospinal fluid cell-free DNA in leptomeningeal metastases of EGFR-mutant non–small-cell lung cancer: a new medium of liquid biopsy. Ann Oncol. 2016;27:1945–50.
Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–92.
Wan JCM, Massie C, Garcia-Corbacho J, Mouliere F, Brenton JD, Caldas C. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17:223–38.
Lam VK, Zhang J, Wu CC, Tran HT, Li L, Diao L, et al. Genotype-specific differences in circulating tumor DNA levels in advanced NSCLC. J Thorac Oncol. 2021;16:601–9.
Laguna JC, Pastor B, Nalda I, Hijazo-Pechero S, Teixido C, Potrony M, et al. Incidental pathogenic germline alterations detected through liquid biopsy in patients with solid tumors: prevalence, clinical utility and implications. Br J Cancer. 2024;130:1420–31.
Hong TH, Hwang S, Dasgupta A, Abbosh C, Hung T, Bredno J, et al. Clinical utility of tumor-naive presurgical circulating tumor DNA detection in early-stage NSCLC. J Thorac Oncol. 2024;19:1512–24.
De Mattos-Arruda L, Mayor R, Ng CKY, Weigelt B, Martínez-Ricarte F, Torrejon D, et al. Cerebrospinal fluid-derived circulating tumour DNA better represents the genomic alterations of brain tumours than plasma. Nat Commun. 2015;6:8839.
Pentsova EI, Shah RH, Tang J, Boire A, Lin X, Kim S, et al. Evaluating cancer of the central nervous system through next-generation sequencing of cerebrospinal fluid. J Clin Oncol. 2016;34:2404–15.
Wang Y, Springer S, Zhang M, McMahon KW, Kinde I, Dobbyn L, et al. Detection of tumor-derived DNA in cerebrospinal fluid of patients with primary tumors of the brain and spinal cord. Proc Natl Acad Sci. 2015;112:9704–9.
Pan W, Gu W, Nagpal S, Gephart MH, Quake SR. Brain tumor mutations detected in cerebral spinal fluid. Clin Chem. 2015;61:514–22.
Wang X, Bai H, Zhang J, Wang Z, Duan J, Cai H, et al. Genetic intratumor heterogeneity remodels the immune microenvironment and induces immune evasion in brain metastasis of lung cancer. J Thorac Oncol. 2024;19:252–72.
Blumenthal DT, Fink KL. Liquid biopsy for assessment of response and resistance in primary brain tumors: challenges and prospects. Mol Diagn Ther. 2015;19:339–51.
Stallard S, Savelieff MG, Wierzbicki K, Bielas JH. Circulating tumor DNA in cerebrospinal fluid: a window into brain tumor genetic heterogeneity. Oncotarget. 2016;7:44777–90.
Piccioni DE, Achrol AS, Kharbanda S, Liebes L, Neuhaus J, Cabral M, et al. Analysis of cell-free circulating tumor DNA in cerebrospinal fluid as a surrogate for medulloblastoma tumor biopsies. Oncotarget. 2019;10:551–60.
Icard P, Simula L, Fournel L, Leroy K, Lupo A, Damotte D, et al. The strategic roles of four enzymes in the interconnection between metabolism and oncogene activation in non-small cell lung cancer: therapeutic implications. Drug Resist Updat. 2022;63:100852.
Fabbri L, Chakraborty A, Robert C, Vagner S. The plasticity of mRNA translation during cancer progression and therapy resistance. Nat Rev Cancer. 2021;21:558–77.
Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501:355–64.
McGranahan N, Swanton C. Clonal heterogeneity and tumor evolution: past, present, and the future. Cell. 2017;168:613–28.
Siravegna G, Marsoni S, Siena S, Bardelli A. Integrating liquid biopsies into the management of cancer. Nat Rev Clin Oncol. 2017;14:531–48.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15:81–94.
Nayak L, Bettegowda C, Scherer F, Galldiks N, Ahluwalia M, Baraniskin A, et al. Liquid biopsy for improving diagnosis and monitoring of CNS lymphomas: a RANO review. Neuro Oncol. 2024;26:993–1011.
Kojic M, Maybury MK, Waddell N, Koufariotis LT, Addala V, Millar A, et al. Efficient detection and monitoring of pediatric brain malignancies with liquid biopsy based on patient-specific somatic mutation screening. Neuro Oncol. 2023;25:1507–17.
Pentsova E. Applications of cerebrospinal fluid circulating tumor cells and circulating tumor-derived DNA in diagnosis, prognosis, and personalized treatment of CNS metastases. Front Oncol. 2024;14:1409383.
Wu J, Liu Z, Huang T, Wang Y, Song MM, Song T, et al. Cerebrospinal fluid circulating tumor DNA depicts profiling of brain metastasis in NSCLC. Mol Oncol. 2023;17:810–24.
Pathakumari B, Prabhavathi M, Anbarasu D, Paramanandhan P, Raja A. Dynamic IgG antibody response to immunodominant antigens of M. tuberculosis for active TB diagnosis in high endemic settings. Clin Chim Acta. 2016;461:25–33.
Zhitnyuk YV, Koval AP, Alferov AA, Shtykova YA, Mamedov IZ, Kushlinskii NE, et al. Deep cfDNA fragment end profiling enables cancer detection. Mol Cancer. 2022;21:26.
Miller AM, Shah RH, Pentsova EI, Pourmaleki M, Briggs S, Distefano N, et al. Tracking tumour evolution in glioma through liquid biopsies of cerebrospinal fluid. Nature. 2019;565:654–8.
Remon J, Besse B, Aix SP, Callejo A, Al-Rabi K, Bernabe R, et al. Osimertinib treatment based on plasma T790M monitoring in patients with EGFR-mutant non-small-cell lung cancer (NSCLC): EORTC Lung Cancer Group 1613 APPLE phase II randomized clinical trial. Ann Oncol. 2023;34:468–76.
Best MG, Wesseling P, Wurdinger T. Tumor-educated platelets as a noninvasive biomarker source for cancer detection and progression monitoring. Cancer Res. 2018;78:3407–12.
Jiang T, et al. Oncogenic drivers in brain metastases of non-small-cell lung cancer: Implications for clinical practice. J Cancer Res Clin Oncol. 2019;145:291–300.
Olsson B, Lautner R, Andreasson U, Ohrfelt A, Portelius E, Bjerke M, et al. CSF and blood biomarkers for the diagnosis of Alzheimer’s disease: a systematic review and meta-analysis. Lancet Neurol. 2016;15:673–84.
Abbosh C, Birkbak NJ, Wilson GA, Jamal-Hanjani M, Constantin T, Salari R, et al. Phylogenetic ctDNA analysis depicts early-stage lung cancer evolution. Nature. 2017;545:446–51.
Diehl F, Schmidt K, Choti MA, Romans K, Goodman S, Li M, et al. Circulating mutant DNA to assess tumor dynamics. Nat Med. 2008;14:985–90.
Bosse Y, Dasgupta A, Abadier M, Guthrie V, Song F, Saavedra Armero V, et al. Prognostic implication of methylation-based circulating tumor DNA detection prior to surgery in stage I non-small cell lung cancer. Cancer Lett. 2024;594:216984.
Acknowledgements
We gratefully acknowledge Prof. Xun Jin and Prof. Manqing Cao for their discussions and advice.
Funding
This work was supported by the China Postdoctoral Science Foundation (grant no. 2024M762383) and the National Natural Science Foundation of China (grant no. 82103642, 82073276, 82273100, 82302999, 82272639).
Author information
Authors and Affiliations
Contributions
ZZ, MQC, and JYZ analysed the data; XYJ, PL, WLL, ZFS, CHS, QL, YZP, PW, LWL, YLL, KKY LM and QY provided methodology support and performed data validation; ZZ wrote the manuscript; XGW, XJ, and MQC supervised the study. All authors have read and agreed to the published version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval and consent to participate
All patient samples were collected with informed consent and approved by the Medical Ethical Committee of Tianjin Medical University Cancer Institute and Hospital (bc20250569). Study participants gave informed consent and provided written consent before tissue collection. Separate written informed consent was obtained from all participants whose identifiable images (e.g., radiological images) were included in this study. This consent explicitly permits the publication of the images in print and online formats. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
All authors consented to the publication.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, Z., Jin, X., Yin, Q. et al. Cyst fluid ctDNA as a biomarker for genetic profiling and treatment monitoring in cystic brain metastases. Br J Cancer (2025). https://doi.org/10.1038/s41416-025-03047-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41416-025-03047-9