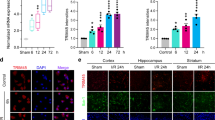
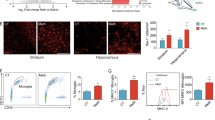
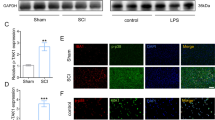
Abstract
Persistent neuroinflammation and progressive neuronal loss are defining features of acute brain injury including traumatic brain injury (TBI) and cerebral stroke. Microglia, the most abundant type of brain-resident immune cells, continuously surveil the environment and play a central role in shaping the inflammatory state of the central nervous system (CNS). In the study, we discovered that the protein expression of METTL3 (a m6A methyltransferase) was upregulated in inflammatory microglia independent of increased Mettl3 gene transcription following TBI in both human and mouse subjects. Subsequently, we identified TRIP12, a HECT-___domain E3 ubiquitin ligase, as a negative regulator of METTL3 protein expression by facilitating METTL3 K48-linked polyubiquitination. Importantly, selective ablation of Mettl3 inhibited microglial pathogenic activities, diminished neutrophil infiltration, rescued neuronal loss and facilitated functional recovery post-TBI. Using MeRIP-seq and CUT&Tag sequencing, we identified that METTL3 promoted the expression of Basic Leucine Zipper Transcriptional Factor ATF-Like (BATF), which in turn directly bound to a cohort of characteristic inflammatory cytokines and chemokine genes. Enhanced activities of BATF in microglia elicited TNF-dependent neurotoxicity and can also promote neutrophil recruitment through releasing CXCL2. Pharmacological inhibition of METTL3 using a BBB-penetrating drug-loaded nano-system showed satisfactory therapeutic effects in both TBI and stroke mouse models. Collectively, our findings identified METTL3-m6A-BATF axis as a potential therapeutic target for terminating detrimental neuroinflammation and progressive neuronal loss following acute brain injury.

METTL3 protein is significantly up-regulated in inflammatory microglia due to the decreased proteasomal degradation mediated by TRIP12 and ERK-USP5 pathways. METTL3 stabilized BATF mRNA stability and promoted BATF expression through the m6A-IGF2BP2-dependent mechanism. Elevated expression of BATF elicits a pro-inflammatory gene program in microglia, and aggravates neuroinflammatory response including local immune responses and peripheral immune cell infiltration. Genetic deletion or pharmaceutically targeting METTL3-BATF axis suppressed microglial pro-inflammatory activities and promoted neurological recovery following TBI and stroke.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Prinz M, Masuda T, Wheeler MA, Quintana FJ. Microglia and central nervous system-associated macrophages-from origin to disease modulation. Annu Rev Immunol. 2021;39:251–77.
Crapser JD, Arreola MA, Tsourmas KI, Green KN. Microglia as hackers of the matrix: sculpting synapses and the extracellular space. Cell Mol Immunol. 2021;18:2472–88.
Vecchiarelli HA, Tremblay M. Microglial transcriptional signatures in the central nervous system: toward a future of unraveling their function in health and disease. Annu Rev Genet. 2023;57:65–86.
Silvin A, Qian J, Ginhoux F. Brain macrophage development, diversity and dysregulation in health and disease. Cell Mol Immunol. 2023;20:1277–89.
Borst K, Dumas AA, Prinz M. Microglia: Immune and non-immune functions. Immunity. 2021;54:2194–208.
Keane L, Antignano I, Riechers SP, Zollinger R, Dumas AA, Offermann N, et al. mTOR-dependent translation amplifies microglia priming in aging mice. J Clin Investig. 2021;131:e132727.
Prinz M, Jung S, Priller J. Microglia biology: one century of evolving concepts. Cell. 2019;179:292–311.
Castro-Sánchez S, García-Yagüe ÁJ, Kügler S, Lastres-Becker I. CX3CR1-deficient microglia shows impaired signalling of the transcription factor NRF2: Implications in tauopathies. Redox Biol. 2019;22:101118.
Islam A, Choudhury ME, Kigami Y, Utsunomiya R, Matsumoto S, Watanabe H, et al. Sustained anti-inflammatory effects of TGF-β1 on microglia/macrophages. Biochim Biophys Acta Mol Basis Dis. 2018;1864:721–34.
Shi K, Zhang J, Dong JF, Shi FD. Dissemination of brain inflammation in traumatic brain injury. Cell Mol Immunol. 2019;16:523–30.
Willis EF, MacDonald KPA, Nguyen QH, Garrido AL, Gillespie ER, Harley SBR, et al. Repopulating microglia promote brain repair in an IL-6-dependent manner. Cell. 2020;180:833–46.e16.
Chen S, Dong Z, Cheng M, Zhao Y, Wang M, Sai N, et al. Homocysteine exaggerates microglia activation and neuroinflammation through microglia localized STAT3 overactivation following ischemic stroke. J Neuroinflamm. 2017;14:187.
Greenhalgh AD, David S, Bennett FC. Immune cell regulation of glia during CNS injury and disease. Nat Rev Neurosci. 2020;21:139–52.
Jin X, Yamashita T. Microglia in central nervous system repair after injury. J Biochem. 2016;159:491–6.
Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–33.
Holtman IR, Skola D, Glass CK. Transcriptional control of microglia phenotypes in health and disease. J Clin Investig. 2017;127:3220–9.
Wu Z, Shi Y, Lu M, Song M, Yu Z, Wang J, et al. METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA. Nucleic Acids Res. 2020;48:11083–96.
Zhou C, Molinie B, Daneshvar K, Pondick JV, Wang J, Van Wittenberghe N, et al. Genome-wide maps of m6A circRNAs identify widespread and cell-type-specific methylation patterns that are distinct from mRNAs. Cell Rep. 2017;20:2262–76.
Mathiyalagan P, Adamiak M, Mayourian J, Sassi Y, Liang Y, Agarwal N, et al. FTO-dependent N(6)-methyladenosine regulates cardiac function during remodeling and repair. Circulation. 2019;139:518–32.
Dorn LE, Lasman L, Chen J, Xu X, Hund TJ, Medvedovic M, et al. The N(6)-methyladenosine mRNA methylase METTL3 controls cardiac homeostasis and hypertrophy. Circulation. 2019;139:533–45.
Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol Cell. 2019;74:640–50.
Liu T, Wei Q, Jin J, Luo Q, Liu Y, Yang Y, et al. The m6A reader YTHDF1 promotes ovarian cancer progression via augmenting EIF3C translation. Nucleic Acids Res. 2020;48:3816–31.
Wang JN, Wang F, Ke J, Li Z, Xu CH, Yang Q, et al. Inhibition of METTL3 attenuates renal injury and inflammation by alleviating TAB3 m6A modifications via IGF2BP2-dependent mechanisms. Sci Transl Med. 2022;14:eabk2709.
Ma S, Sun B, Duan S, Han J, Barr T, Zhang J, et al. YTHDF2 orchestrates tumor-associated macrophage reprogramming and controls antitumor immunity through CD8(+) T cells. Nat Immunol. 2023;24:255–66.
Weng H, Huang F, Yu Z, Chen Z, Prince E, Kang Y, et al. The m(6)A reader IGF2BP2 regulates glutamine metabolism and represents a therapeutic target in acute myeloid leukemia. Cancer Cell. 2022;40:1566–82.e10.
Zhang X, Yin H, Zhang X, Jiang X, Liu Y, Zhang H, et al. N6-methyladenosine modification governs liver glycogenesis by stabilizing the glycogen synthase 2 mRNA. Nat Commun. 2022;13:7038.
Pham D, Silberger DJ, Nguyen KN, Gao M, Weaver CT, Hatton RD. Batf stabilizes Th17 cell development via impaired Stat5 recruitment of Ets1-Runx1 complexes. Embo J. 2023;42:e109803.
Zhang X, Zhang C, Qiao M, Cheng C, Tang N, Lu S, et al. Depletion of BATF in CAR-T cells enhances antitumor activity by inducing resistance against exhaustion and formation of central memory cells. Cancer Cell. 2022;40:1407–22.e7.
Huang TY, Hirota M, Sasaki D, Kalra RS, Chien HC, Tamai M, et al. Phosphoenolpyruvate regulates the Th17 transcriptional program and inhibits autoimmunity. Cell Rep. 2023;42:112205.
Wu X, Khatun A, Kasmani MY, Chen Y, Zheng S, Atkinson S, et al. Group 3 innate lymphoid cells require BATF to regulate gut homeostasis in mice. J Exp Med. 2022;219:e20211861.
Bae S, Kim K, Kang K, Kim H, Lee M, Oh B, et al. RANKL-responsive epigenetic mechanism reprograms macrophages into bone-resorbing osteoclasts. Cell Mol Immunol. 2023;20:94–109.
Choi JK, Yu CR, Bing SJ, Jittayasothorn Y, Mattapallil MJ, Kang M, et al. IL-27-producing B-1a cells suppress neuroinflammation and CNS autoimmune diseases. Proc Natl Acad Sci USA. 2021;118:e2109548118.
Gosselin D, Skola D, Coufal NG, Holtman IR, Schlachetzki JCM, Sajti E, et al. An environment-dependent transcriptional network specifies human microglia identity. Science. 2017;356:eaal3222.
Zheng P, Zhang N, Ren D, Yu C, Zhao B, Zhang Y. Integrated spatial transcriptome and metabolism study reveals metabolic heterogeneity in human injured brain. Cell Rep. Med. 2023;4:101057.
Yang Z, Lin P, Chen B, Zhang X, Xiao W, Wu S, et al. Autophagy alleviates hypoxia-induced blood-brain barrier injury via regulation of CLDN5 (claudin 5). Autophagy. 2021;17:3048–67.
Luo J, Wu X, Liu H, Cui W, Guo W, Guo K, et al. Antagonism of protease-activated receptor 4 protects against traumatic brain injury by suppressing neuroinflammation via inhibition of Tab2/NF-κB signaling. Neurosci Bull. 2021;37:242–54.
Yang L, Han B, Zhang Z, Wang S, Bai Y, Zhang Y, et al. Extracellular vesicle-mediated delivery of circular RNA SCMH1 promotes functional recovery in rodent and nonhuman primate ischemic stroke models. Circulation. 2020;142:556–74.
Gao XQ, Zhang YH, Liu F, Ponnusamy M, Zhao XM, Zhou LY, et al. The piRNA CHAPIR regulates cardiac hypertrophy by controlling METTL3-dependent N(6)-methyladenosine methylation of Parp10 mRNA. Nat Cell Biol. 2020;22:1319–31.
Zhang S, Wu X, Wang J, Shi Y, Hu Q, Cui W, et al. Adiponectin/AdiopR1 signaling prevents mitochondrial dysfunction and oxidative injury after traumatic brain injury in a SIRT3 dependent manner. Redox Biol. 2022;54:102390.
Sun HL, Zhu AC, Gao Y, Terajima H, Fei Q, Liu S, et al. Stabilization of ERK-phosphorylated METTL3 by USP5 increases m(6)A methylation. Mol Cell. 2020;80:633–47.e7.
Shin MK, Vázquez-Rosa E, Koh Y, Dhar M, Chaubey K, Cintrón-Pérez CJ, et al. Reducing acetylated tau is neuroprotective in brain injury. Cell. 2021;184:2715–32.e23.
Luo S, Liao C, Zhang L, Ling C, Zhang X, Xie P, et al. METTL3-mediated m6A mRNA methylation regulates neutrophil activation through targeting TLR4 signaling. Cell Rep. 2023;42:112259.
Chen Y, Zander RA, Wu X, Schauder DM, Kasmani MY, Shen J, et al. BATF regulates progenitor to cytolytic effector CD8(+) T cell transition during chronic viral infection. Nat Immunol. 2021;22:996–1007.
Seo H, González-Avalos E, Zhang W, Ramchandani P, Yang C, Lio CJ, et al. BATF and IRF4 cooperate to counter exhaustion in tumor-infiltrating CAR T cells. Nat Immunol. 2021;22:983–95.
Pham D, Moseley CE, Gao M, Savic D, Winstead CJ, Sun M, et al. Batf pioneers the reorganization of chromatin in developing effector T cells via Ets1-dependent recruitment of Ctcf. Cell Rep. 2019;29:1203–20.e7.
Jiang S, Willox B, Zhou H, Holthaus AM, Wang A, Shi TT, et al. Epstein-Barr virus nuclear antigen 3C binds to BATF/IRF4 or SPI1/IRF4 composite sites and recruits Sin3A to repress CDKN2A. Proc Natl Acad Sci USA. 2014;111:421–6.
Ndoja A, Reja R, Lee SH, Webster JD, Ngu H, Rose CM, et al. Ubiquitin ligase COP1 suppresses neuroinflammation by degrading c/EBPβ in microglia. Cell. 2020;182:1156–69.e12.
Wu X, Kasmani MY, Zheng S, Khatun A, Chen Y, Winkler W, et al. BATF promotes group 2 innate lymphoid cell-mediated lung tissue protection during acute respiratory virus infection. Sci Immunol. 2022;7:eabc9934.
Wang T, Zhou Y, Zhou Z, Zhang P, Yan R, Sun L, et al. Secreted protease PRSS35 suppresses hepatocellular carcinoma by disabling CXCL2-mediated neutrophil extracellular traps. Nat Commun. 2023;14:1513.
McDowell SAC, Milette S, Doré S, Yu MW, Sorin M, Wilson L, et al. Obesity alters monocyte developmental trajectories to enhance metastasis. J Exp Med. 2023;220:e20220509.
Yankova E, Blackaby W, Albertella M, Rak J, De Braekeleer E, Tsagkogeorga G, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601.
Kim DG, Bynoe MS. A2A adenosine receptor modulates drug efflux transporter P-glycoprotein at the blood-brain barrier. J Clin Investig. 2016;126:1717–33.
Zhang C, Jiang M, Wang WQ, Zhao SJ, Yin YX, Mi QJ, et al. Selective mGluR1 negative allosteric modulator reduces blood-brain barrier permeability and cerebral edema after experimental subarachnoid hemorrhage. Transl Stroke Res. 2020;11:799–811.
Han B, Jiang W, Cui P, Zheng K, Dang C, Wang J, et al. Microglial PGC-1α protects against ischemic brain injury by suppressing neuroinflammation. Genome Med. 2021;13:47.
Funding
This work was supported by the State Key Program of the National Natural Science Foundation of China (Nos. 81630027 and 82130038, to YQ). And the authors would like to thank to Clinical Research Center for Neurosurgical Diseases of Shaanxi Province and Shaanxi International Science & Technology Cooperation Base for their support.
Author information
Authors and Affiliations
Contributions
YQ, SG and WG designed the study. XW, HL, SZ, and WC performed animal studies. JW, QH and YS performed the in vitro experiments. HB and LL were responsible for human clinical studies. The images were photographed by YW and TZ. JL, PZ, DF, and LH helped to analyze MeRIP-seq, RNA-seq and CUT&Tag data. XW, HL, and JW wrote the manuscript. SG revised the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethics
All animal experimental procedures were approved by the Ethics Committee of the Fourth Military Medical University (IACUC- 20210556) and performed according to the Animal Research: Reporting in Vivo Experiments (ARRIVE) guidelines. All procedures involving the participants were granted ethical approval by the Ethics Committee of Tangdu Hospital, Fourth Military Medical University (TD-202103–006). Furthermore, the human research conducted in this study adhered to the principles outlined in the Helsinki Declaration, and written informed consent was obtained from each patient or their legal surrogate.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wu, X., Liu, H., Wang, J. et al. The m6A methyltransferase METTL3 drives neuroinflammation and neurotoxicity through stabilizing BATF mRNA in microglia. Cell Death Differ 32, 100–117 (2025). https://doi.org/10.1038/s41418-024-01329-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41418-024-01329-y