Abstract
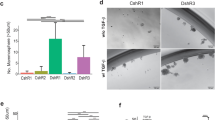
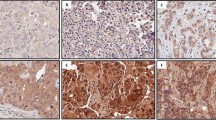
Breast cancer is a heterogeneous disease. In particular, triple-negative breast cancer (TNBC) comprises various molecular subgroups with unclear identities and currently has few targeted treatment options. Our previous study identified protein C receptor (Procr) as a surface marker on mammary stem cells (MaSCs) located in the basal layer of the normal mammary gland. Given the possible connection of TNBC with basal layer stem cells, we conducted comparative analyses of Procr in breast cancers of mouse and human origin. In mouse mammary tumors, we showed that Procr+ cells are enriched for cancer stem cells (CSCs) in Wnt1 basal-like tumors, but not in Brca1 basal-like tumors or PyVT luminal tumors. In human cancers, PROCR was robustly expressed in half of TNBC cases. Experiments with patient-derived xenografts (PDXs) revealed that PROCR marks CSCs in this discrete subgroup (referred to as PROCR+ TNBC). Interfering with the function of PROCR using an inhibitory nanobody reduced the CSC numbers, arrested tumor growth and prevented rapid tumor recurrence. Our data suggest a key role of MaSC in breast tumorigenesis. Moreover, our work indicates that PROCR can be used as a biomarker to stratify TNBC into clinically relevant subgroups and may provide a novel targeted treatment strategy for this clinically important tumor subtype.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Foulkes, W. D., Smith, I. E. & Reis-Filho, J. S. Triple-negative breast cancer. N. Engl. J. Med. 363, 1938–1948 (2010).
Lehmann, B. D. et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J. Clin. Invest. 121, 2750–2767 (2011).
Metzger-Filho, O. et al. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. 30, 1879–1887 (2012).
Carey, L., Winer, E., Viale, G., Cameron, D. & Gianni, L. Triple-negative breast cancer: disease entity or title of convenience? Nat. Rev. Clin. Oncol. 7, 683–692 (2010).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Prat, A. et al. Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer. Breast Cancer Res. 12, R68 (2010).
Adamo, B. & Anders, C. K. Stratifying triple-negative breast cancer: which definition(s) to use? Breast Cancer Res. 13, 105 (2011).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10, 717–728 (2012).
Magee, J. A., Piskounova, E. & Morrison, S. J. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell 21, 283–296 (2012).
Nguyen, L. V., Vanner, R., Dirks, P. & Eaves, C. J. Cancer stem cells: an evolving concept. Nat. Rev. Cancer 12, 133–143 (2012).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Ginestier, C. et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell 1, 555–567 (2007).
Meyer, M. J. et al. CD44posCD49fhiCD133/2hi defines xenograft-initiating cells in estrogen receptor-negative breast cancer. Cancer Res. 70, 4624–4633 (2010).
Wang, D. et al. Identification of multipotent mammary stem cells by protein C receptor expression. Nature 517, 81–84 (2015).
Li, Y. et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl Acad. Sci. USA 100, 15853–15858 (2003).
Tsukamoto, A. S., Grosschedl, R., Guzman, R. C., Parslow, T. & Varmus, H. E. Expression of the int-1 gene in transgenic mice is associated with mammary gland hyperplasia and adenocarcinomas in male and female mice. Cell 55, 619–625 (1988).
Herschkowitz, J. I. et al. Identification of conserved gene expression features between murine mammary carcinoma models and human breast tumors. Genome Biol. https://doi.org/10.1186/Gb-2007-8-5-R76 (2007).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell Biol. 12, 954–961 (1992).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nat. Genet 22, 37–43 (1999).
Cleary, A. S., Leonard, T. L., Gestl, S. A. & Gunther, E. J. Tumour cell heterogeneity maintained by cooperating subclones in Wnt-driven mammary cancers. Nature 508, 113–117 (2014).
Li, Y., Hively, W. P. & Varmus, H. E. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene 19, 1002–1009 (2000).
Shipitsin, M. et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 11, 259–273 (2007).
Wang, D. et al. Protein C receptor stimulates multiple signaling pathways in breast cancer cells. J. Biol. Chem. 293, 1413–1424 (2018).
Neve, R. M. et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006).
Gilbert, L. A. et al. CRISPR-mediated modular RNA-guided regulation of transcription in eukaryotes. Cell 154, 442–451 (2013).
Hamers-Casterman, C. et al. Naturally occurring antibodies devoid of light chains. Nature 363, 446–448 (1993).
Tice, D. A., Biscardi, J. S., Nickles, A. L. & Parsons, S. J. Mechanism of biological synergy between cellular Src and epidermal growth factor receptor. Proc. Natl Acad. Sci. USA 96, 1415–1420 (1999).
Hwang-Verslues, W. W. et al. Multiple lineages of human breast cancer stem/progenitor cells identified by profiling with stem cell markers. PLoS ONE 4, e8377 (2009).
Schaffner, F. et al. Endothelial protein C receptor function in murine and human breast cancer development. PLoS ONE 8, e61071 (2013).
Kreso, A. & Dick, J. E. Evolution of the cancer stem cell model. Cell Stem Cell 14, 275–291 (2014).
Clarke, M. F. et al. Cancer stem cells–perspectives on current status and future directions: AACR Workshop on cancer stem cells. Cancer Res. 66, 9339–9344 (2006).
Chakrabarti, R. et al. DeltaNp63 promotes stem cell activity in mammary gland development and basal-like breast cancer by enhancing Fzd7 expression and Wnt signalling. Nat. Cell Biol. 16, 1001–1013 (2014).
Zhang, M. et al. Identification of tumor-initiating cells in a p53-null mouse model of breast cancer. Cancer Res. 68, 4674–4682 (2008).
Su, X. et al. TAp63 suppresses mammary tumorigenesis through regulation of the Hippo pathway. Oncogene, https://doi.org/10.1038/onc.2016.388 (2016).
Morel, A. P. et al. A stemness-related ZEB1-MSRB3 axis governs cellular pliancy and breast cancer genome stability. Nat. Med 23, 568–578 (2017).
Lindeman, G. J. & Visvader, J. E. Insights into the cell of origin in breast cancer and breast cancer stem cells. Asia Pac. J. Clin. Oncol. 6, 89–97 (2010).
Lim, E. et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat. Med. 15, 907–913 (2009).
Molyneux, G. et al. BRCA1 basal-like breast cancers originate from luminal epithelial progenitors and not from basal stem cells. Cell Stem Cell 7, 403–417 (2010).
Proia, T. A. et al. Genetic predisposition directs breast cancer phenotype by dictating progenitor cell fate. Cell Stem Cell 8, 149–163 (2011).
Nolan, E. et al. RANK ligand as a potential target for breast cancer prevention in BRCA1-mutation carriers. Nat. Med. 22, 933–939 (2016).
Sau, A. et al. Persistent activation of NF-kappaB in BRCA1-deficient mammary progenitors drives aberrant proliferation and accumulation of DNA damage. Cell Stem Cell 19, 52–65 (2016).
Day, C. P., Merlino, G. & Van Dyke, T. Preclinical mouse cancer models: a maze of opportunities and challenges. Cell 163, 39–53 (2015).
Ye, F. G. et al. Cytidine deaminase axis modulated by miR-484 differentially regulates cell proliferation and chemoresistance in breast cancer. Cancer Res. 75, 1504–1515 (2015).
Acknowledgements
We are grateful to Drs. Chi-Chung Hui, Esther Verheyen and Roel Nusse for critical reading of the manuscript. We thank Drs. Dangsheng Li and Weiguo Zou for helpful discussion. We thank Dr. Suling Liu for providing reagents. We thank Dr. Jianhua Sui for advice on antibody development. This work was supported by grants from National Natural Science Foundation of China (31830056, 31861163006, 31625020, 31530045, 31661143043 to Y.A.Z, 81874113 to Z.-M.S; 81672601, 81872137 to X.H.), Chinese Academy of Sciences (XDA12020378 to Y.J. and XDB19000000 to Y.A.Z.), Shanghai Municipal Science and Technology Commission (17XD1404000 to Y.A.Z.) and National Ten Thousand Talents Program (to Y. A. Z.).
Author information
Authors and Affiliations
Contributions
Y.A.Z and Z.-M.S conceived the study. D.W. performed most FACS analyses, genetic, xenograft and in vitro experiments. C.L., R.Y. performed xenograft, antibody and chemo agent administration. D.W., Y.J., C.C. and L.B. performed monoclonal antibody screening and characterization. J.W. performed mouse breeding. X.H., S.L., F.Q., L.Y., L.C. and Z.-M.S provided TMA and PDX samples. Y.B. and Y.-R.L performed bioinformatics analysis. D.W., X.H. and Y.A.Z. analyzed the data and wrote the manuscript. C.L., G.G., H.J., D.L., L.L. and J.C. helped project design and manuscript editing.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, D., Hu, X., Liu, C. et al. Protein C receptor is a therapeutic stem cell target in a distinct group of breast cancers. Cell Res 29, 832–845 (2019). https://doi.org/10.1038/s41422-019-0225-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-019-0225-9
This article is cited by
-
CXCR4+ mammary gland macrophageal niche promotes tumor initiating cell activity and immune suppression during tumorigenesis
Nature Communications (2025)
-
Endothelial protein C receptor promotes retinal neovascularization through heme catabolism
Nature Communications (2025)
-
Cancer stem cells: advances in knowledge and implications for cancer therapy
Signal Transduction and Targeted Therapy (2024)
-
LINC00853 contributes to tumor stemness of gastric cancer through FOXP3-mediated transcription of PDZK1IP1
Biological Procedures Online (2023)
-
Protein C receptor maintains cancer stem cell properties via activating lipid synthesis in nasopharyngeal carcinoma
Signal Transduction and Targeted Therapy (2022)