Abstract
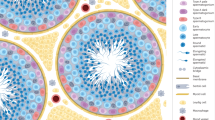
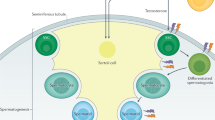
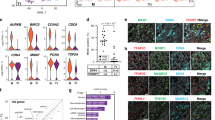
Unlike most organs that mature during the fetal period, the male reproductive system reaches maturity only at puberty with the commencement of spermatogenesis. Robust modelling of human testicular organogenesis in vitro would facilitate research into mechanisms of and factors affecting human spermatogenic failure and male fertility preservation in prepubertal tumor patients. Here, we report successful recapitulation of human testicular organogenesis in vitro from fetal gonadal ridge. Our model displayed the formation of mature seminiferous epithelium and self-renewing spermatogonia. Remarkably, in vitro-derived haploid spermatids have undergone meiotic recombination, and showed increased genetic diversity as indicated by genetic analysis. Moreover, these spermatids were able to fertilize oocytes and support subsequent blastocyst formation. The in vitro testicular organogenesis system described here will play an important role in elucidating the regulation of human testis development and maintaining male fertility in prepubertal cancer patients.
Similar content being viewed by others
Login or create a free account to read this content
Gain free access to this article, as well as selected content from this journal and more on nature.com
or
References
Chemes, H. E. Infancy is not a quiescent period of testicular development. Int. J. Androl. 24, 2–7 (2001).
Rey, R. A., Musse, M., Venara, M. & Chemes, H. E. Ontogeny of the androgen receptor expression in the fetal and postnatal testis: its relevance on Sertoli cell maturation and the onset of adult spermatogenesis. Microsc. Res. Tech. 72, 787–795 (2009).
Stukenborg, J. B., Colon, E. & Soder, O. Ontogenesis of testis development and function in humans. Sex. Dev. 4, 199–212 (2010).
Clermont, Y. Kinetics of spermatogenesis in mammals: seminiferous epithelium cycle and spermatogonial renewal. Physiol. Rev. 52, 198–236 (1972).
Inhorn, M. C. & Patrizio, P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum. Reprod. Update 21, 411–426 (2015).
Stiller, C. A. & Parkin, D. M. Geographic and ethnic variations in the incidence of childhood cancer. Br. Med. Bull. 52, 682–703 (1996).
Hargreave, M. et al. Association between fertility treatment and cancer risk in children. JAMA 322, 2203–2210 (2019).
Thomson, A. B. et al. Semen quality and spermatozoal DNA integrity in survivors of childhood cancer: a case-control study. Lancet 360, 361–367 (2002).
Ginsberg, J. P. Educational paper: the effect of cancer therapy on fertility, the assessment of fertility and fertility preservation options for pediatric patients. Eur. J. Pediatrics 170, 703–708 (2011).
Martinez, G. et al. Impact of Hodgkin or non-Hodgkin lymphoma and their treatments on sperm aneuploidy: a prospective study by the French CECOS network. Fertil. Steril. 107, 341–350 e345 (2017).
Chatterjee, R., Mills, W., Katz, M., McGarrigle, H. H. & Goldstone, A. H. Germ cell failure and Leydig cell insufficiency in post-pubertal males after autologous bone marrow transplantation with BEAM for lymphoma. Bone Marrow Transplant. 13, 519–522 (1994).
Ji, D. et al. Somatic mutations and immune alternation in rectal cancer following neoadjuvant chemoradiotherapy. Cancer Immunol. Res. 6, 1401–1416 (2018).
Rakici, S. Y., Bedir, R. & Hatipoglu, C. Are there predictors that can determine neoadjuvant treatment responses in rectal cancer? Turkish J. Gastroenterol. https://doi.org/10.5152/tjg.2018.18179 (2018).
Lee, I. H. et al. Genetic variations using whole-exome sequencing might predict response for neoadjuvant chemoradiotherapy in locally advanced rectal cancer. Med. Oncol. 35, 145 (2018).
de Lambert, G., Poirot, C., Guerin, F., Brugieres, L. & Martelli, H. Preservation of fertility in children with cancer. Bull. du cancer 102, 436–442 (2015).
Levine, J. Fertility preservation in children and adolescents with cancer. Minerva Pediatrica 63, 49–59 (2011).
Mitchell, R. T., Saunders, P. T., Sharpe, R. M., Kelnar, C. J. & Wallace, W. H. Male fertility and strategies for fertility preservation following childhood cancer treatment. Endocr. Dev. 15, 101–134 (2009).
Tournaye, H., Dohle, G. R. & Barratt, C. L. Fertility preservation in men with cancer. Lancet 384, 1295–1301 (2014).
Handel, M. A., Eppig, J. J. & Schimenti, J. C. Applying “gold standards” to in-vitro-derived germ cells. Cell 157, 1257–1261 (2014).
Tang, W. W. et al. A unique gene regulatory network resets the human germline epigenome for development. Cell 161, 1453–1467 (2015).
Li, L. et al. Single-cell RNA-Seq analysis mmaps development of human germline cells and gonadal niche interactions. Cell Stem Cell 20, 858–873 e854 (2017).
Costoya, J. A. et al. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat. Genet. 36, 653–659 (2004).
Sasaki, K. et al. The germ cell fate of Cynomolgus monkeys is specified in the Nascent Amnion. Dev. Cell 39, 169–185 (2016).
Sato, T. et al. In vitro production of functional sperm in cultured neonatal mouse testes. Nature 471, 504–507 (2011).
Zhou, Q. et al. Complete meiosis from embryonic stem cell-derived germ cells in vitro. Cell Stem Cell 18, 330–340 (2016).
Nistal, M., Abaurrea, M. A. & Paniagua, R. Morphological and histometric study on the human Sertoli cell from birth to the onset of puberty. J. Anat. 134, 351–363 (1982).
Habert, R., Lejeune, H. & Saez, J. M. Origin, differentiation and regulation of fetal and adult Leydig cells. Mol. Cell. Endocrinol. 179, 47–74 (2001).
Syrjanen, J. L., Pellegrini, L. & Davies, O. R. A molecular model for the role of SYCP3 in meiotic chromosome organisation. eLife 3, https://doi.org/10.7554/eLife.02963 (2014).
Nakano, T. et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell 10, 771–785 (2012).
Lancaster, M. A. et al. Cerebral organoids model human brain development and microcephaly. Nature 501, 373–379 (2013).
Takebe, T. et al. Vascularized and functional human liver from an iPSC-derived organ bud transplant. Nature 499, 481–484 (2013).
Gohbara, A. et al. In vitro murine spermatogenesis in an organ culture system. Biol. Reprod. 83, 261–267 (2010).
Reda, A. et al. In vitro differentiation of rat spermatogonia into round spermatids in tissue culture. Mol. Hum. Reprod. 22, 601–612 (2016).
Rey, R. A. Mini-puberty and true puberty: differences in testicular function. Annales d’endocrinologie 75, 58–63 (2014).
Iliadou, P. K., Tsametis, C., Kaprara, A., Papadimas, I. & Goulis, D. G. The sertoli cell: novel clinical potentiality. Hormones 14, 504–514 (2015).
Tracey, M. Short tandem repeat-based identification of individuals and parents. Croatian Med. J. 42, 233–238 (2001).
van der Westerlaken, L., Helmerhorst, F., Dieben, S. & Naaktgeboren, N. Intracytoplasmic sperm injection as a treatment for unexplained total fertilization failure or low fertilization after conventional in vitro fertilization. Fertil. Steril. 83, 612–617 (2005).
Van Landuyt, L. et al. Blastocyst formation in in vitro fertilization versus intracytoplasmic sperm injection cycles: influence of the fertilization procedure. Fertil. Steril. 83, 1397–1403 (2005).
Vloeberghs, V., Verheyen, G. & Tournaye, H. Intracytoplasmic spermatid injection and in vitro maturation: fact or fiction. Clinics 68, 151–156 (2013).
Pongsuthirak, P., Songveeratham, S. & Vutyavanich, T. Comparison of blastocyst and Sage media for in vitro maturation of human immature oocytes. Reprod. Sci. 22, 343–346 (2015).
Kosmac, K. et al. Immunohistochemical identification of human skeletal muscle macrophages. Bio-protocol 8, https://doi.org/10.21769/BioProtoc.2883 (2018).
O’Brien, D. A. Isolation, separation, and short-term culture of spermatogenic cells. Male Reproductive Toxicol. 3A, 246–264 (1993).
Zong, C., Lu, S., Chapman, A. R. & Xie, X. S. Genome-wide detection of single-nucleotide and copy-number variations of a single human cell. Science 338, 1622–1626 (2012).
Acknowledgements
We thank Peng Xiang for the 3β-HSD, CYP17A1 antibodies, Fei Gao for the AMH antibody, and Sigrid Eckardt for help with paper preparation. This work was supported by National Key R&D Program (2017YFA0103803), National Natural Science Foundation of China (31890784), and National Key R&D Program (2016YFA0500903, 2016YFA0503300).
Author information
Authors and Affiliations
Contributions
Conceptualization, X.G., F.D., Q.Z., and J.S.; Methodology, Q.C., Q.Zeng, Y.W., J.T., J.Y.; Investigation, Y.Y., L.L., H.Z., M.H., J.C., Q.Zhou, R.H., X.W., Z.Z., X.Y., J.H., D.H., J.L.; Writing (original draft), X.G., F.D., Q.Z. and J.S.; Writing (review and editing), X.G., F.D., Q.Z., and J.S.; Funding Acquisition, Y.Y., X.G., F.D., Q.Z., and J.S.; Supervision, X.G., F.D., Q.Z., and J.S.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Yuan, Y., Li, L., Cheng, Q. et al. In vitro testicular organogenesis from human fetal gonads produces fertilization-competent spermatids. Cell Res 30, 244–255 (2020). https://doi.org/10.1038/s41422-020-0283-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41422-020-0283-z
This article is cited by
-
In vitro sperm generation from immature mouse testicular tissue using plasma rich in growth factors
Stem Cell Research & Therapy (2025)
-
Endocannabinoid system upregulates the enrichment and differentiation of human iPSC- derived spermatogonial stem cells via CB2R agonism
Biological Research (2025)
-
How the extra X chromosome impairs the development of male fetal germ cells
Nature (2024)
-
Molecular mechanisms of cellular dysfunction in testes from men with non-obstructive azoospermia
Nature Reviews Urology (2024)
-
The Evolutionary Route of in vitro Human Spermatogenesis: What is the Next Destination?
Stem Cell Reviews and Reports (2024)