Abstract
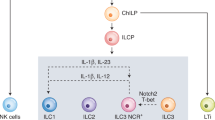
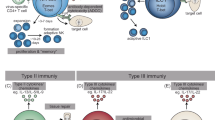
Innate lymphoid cells (ILCs), including natural killer (NK) cells, ILC1s, ILC2s, ILC3s, and lymphoid tissue inducer (LTi) cells, comprise the first line of innate immune defense against pathogens and tumors. Over the past decade, accumulating evidence has demonstrated immunological memory in ILC subsets: for example, NK cells recall haptens, viruses, and cytokines; ILC1s recall haptens; and ILC2s recall cytokines. Although the development and functions of ILCs mirror those of T-cell subsets, ILC and T-cell memory exhibit both common characteristics and specific properties. Encouragingly, ILC memory has been found to confer benefits in long-term tumor control and vaccination, providing insight for novel memory ILC-based tumor immunotherapy and vaccine-development strategies. In this review, we discuss the evidence supporting ILC memory and present a comprehensive framework of the ILC memory system.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Spits, H. et al. Innate lymphoid cells--a proposal for uniform nomenclature. Nat. Rev. Immunol. 13, 145–149 (2013).
Eberl, G., Colonna, M., Di Santo, J. P. & McKenzie, A. N. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science 348, aaa6566 (2015).
Artis, D. & Spits, H. The biology of innate lymphoid cells. Nature 517, 293–301 (2015).
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Fuchs, A. et al. Intraepithelial type 1 innate lymphoid cells are a unique subset of IL-12-and IL-15-responsive IFN-gamma-producing cells. Immunity 38, 769–781 (2013).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Weizman, O. E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell 171, 795–808 (2017). e712.
Moro, K. et al. Innate production of TH2 cytokines by adipose tissue-associated c-Kit+Sca-1+lymphoid cells. Nature 463, 540 (2009).
Neill, D. R. et al. Nuocytes represent a new innate effector leukocyte that mediates type-2 immunity. Nature 464, 1367 (2010).
Cella, M. et al. A human natural killer cell subset provides an innate source of IL-22 for mucosal immunity. Nature 457, 722 (2008).
Cupedo, T. et al. Human fetal lymphoid tissue–inducer cells are interleukin 17–producing precursors to RORC+CD127+natural killer–like cells. Nat. Immunol. 10, 66 (2008).
Luci, C. et al. Influence of the transcription factor RORγt on the development of NKp46+cell populations in gut and skin. Nat. Immunol. 10, 75 (2008).
Satoh-Takayama, N. et al. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity 29, 958–970 (2008).
Mebius, R. E., Rennert, P. & Weissman, I. L. Developing lymph nodes collect CD4+CD3− LTβ+cells that can differentiate to APC, NK cells, and follicular cells but not T or B cells. Immunity 7, 493–504 (1997).
O’Leary, J. G., Goodarzi, M., Drayton, D. L. & von Andrian, U. H. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat. Immunol. 7, 507–516 (2006).
Sun, J. C., Beilke, J. N. & Lanier, L. L. Adaptive immune features of natural killer cells. Nature 457, 557–561 (2009).
Cooper, M. A. et al. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 106, 1915–1919 (2009).
Reeves, R. K. et al. Antigen-specific NK cell memory in rhesus macaques. Nat. Immunol. 16, 927–932 (2015).
Lopez-Verges, S. et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 108, 14725–14732 (2011).
Wang, X. et al. Memory formation and long-term maintenance of IL-7Ralpha(+) ILC1s via a lymph node-liver axis. Nat. Commun. 9, 4854 (2018).
Martinez-Gonzalez, I. et al. Allergen-experienced group 2 innate lymphoid cells acquire memory-like properties and enhance allergic lung inflammation. Immunity 45, 198–208 (2016).
Paust, S. et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat. Immunol. 11, 1127–1135 (2010).
Peng, H. et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 123, 1444–1456 (2013).
Peng, H., Sun, R. & Liver-resident, N. K. cells and their potential functions. Cell. Mol. Immunol. 14, 890 (2017).
Peng, H., Wisse, E. & Tian, Z. Liver natural killer cells: subsets and roles in liver immunity. Cell. Mol. Immunol. 13, 328–336 (2016).
Sojka, D. K. et al. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3, e01659 (2014).
Fan, X. & Rudensky, A. Y. Hallmarks of tissue-resident lymphocytes. Cell 164, 1198–1211 (2016).
Tang, L. et al. Differential phenotypic and functional properties of liver-resident NK cells and mucosal ILC1s. J. Autoimmun. 67, 29–35 (2016).
Yu, Y. et al. Single-cell RNA-seq identifies a PD-1hi ILC progenitor and defines its development pathway. Nature 539, 102–106 (2016).
Constantinides, M. G., McDonald, B. D., Verhoef, P. A. & Bendelac, A. A committed precursor to innate lymphoid cells. Nature 508, 397 (2014).
Zhang, L. H., Shin, J. H., Haggadone, M. D. & Sunwoo, J. B. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J. Exp. Med. 213, 2249–2257 (2016).
van den Boorn, J. G. et al. Inflammasome-dependent induction of adaptive NK cell memory. Immunity 44, 1406–1421 (2016).
Wight, A. et al. Critical role for the Ly49 family of class I MHC receptors in adaptive natural killer cell responses. Proc. Natl. Acad. Sci. USA 115, 11579–11584 (2018).
O’Sullivan, T. E., Sun, J. C. & Lanier, L. L. Natural killer cell memory. Immunity 43, 634–645 (2015).
Nabekura, T. & Lanier, L. L. Antigen-specific expansion and differentiation of natural killer cells by alloantigen stimulation. J. Exp. Med. 211, 2455–2465 (2014).
Nabekura, T. & Lanier, L. L. Activating receptors for self-MHC class I enhance effector functions and memory differentiation of NK cells during mouse cytomegalovirus infection. Immunity 45, 74–82 (2016).
Nabekura, T. et al. Costimulatory molecule DNAM-1 is essential for optimal differentiation of memory natural killer cells during mouse cytomegalovirus infection. Immunity 40, 225–234 (2014).
Sun, J. C. et al. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med. 209, 947–954 (2012).
Madera, S. et al. Type I IFN promotes NK cell expansion during viral infection by protecting NK cells against fratricide. J. Exp. Med. 213, 225–233 (2016).
Madera, S. & Sun, J. C. Cutting edge: stage-specific requirement of IL-18 for antiviral NK cell expansion. J. Immunol. 194, 1408–1412 (2015).
Nabekura, T., Girard, J. P. & Lanier, L. L. IL-33 receptor ST2 amplifies the expansion of NK cells and enhances host defense during mouse cytomegalovirus infection. J. Immunol. 194, 5948–5952 (2015).
Beaulieu, A. M., Zawislak, C. L., Nakayama, T. & Sun, J. C. The transcription factor Zbtb32 controls the proliferative burst of virus-specific natural killer cells responding to infection. Nat. Immunol. 15, 546–553 (2014).
Geary, C. D. et al. Non-redundant ISGF3 components promote NK cell survival in an auto-regulatory manner during viral infection. Cell Rep. 24, 1949–1957 (2018). e1946.
Rapp, M. et al. Core-binding factor β and Runx transcription factors promote adaptive natural killer cell responses. Sci. Immunol. 2, pii: eaan379 (2017).
min-Oo, G., Bezman, N. A., Madera, S., Sun, J. C. & Lanier, L. L. Proapoptotic Bim regulates antigen-specific NK cell contraction and the generation of the memory NK cell pool after cytomegalovirus infection. J. Exp. Med. 211, 1289–1296 (2014).
O’Sullivan, T. E., Johnson, L. R., Kang, H. H. & Sun, J. C. BNIP3- and BNIP3L-mediated mitophagy promotes the generation of natural killer cell memory. Immunity 43, 331–342 (2015).
Bezman, N. A. et al. Molecular definition of the identity and activation of natural killer cells. Nat. Immunol. 13, 1000–1009 (2012).
min-Oo, G. & Lanier, L. L. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J. Exp. Med. 211, 2669–2680 (2014).
Lau, C. M. et al. Epigenetic control of innate and adaptive immune memory. Nat. Immunol. 19, 963–972 (2018).
Rapp, M., Wiedemann, G. M. & Sun, J. C. Memory responses of innate lymphocytes and parallels with T cells. Semin. Immunopathol. 40, 343–355 (2018).
Buck, M. D., O’Sullivan, D. & Pearce, E. L. T cell metabolism drives immunity. J. Exp. Med. 212, 1345–1360 (2015).
Bantug, G. R., Galluzzi, L., Kroemer, G. & Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 18, 19–34 (2018).
Pearce, E. L. et al. Enhancing CD8 T-cell memory by modulating fatty acid metabolism. Nature 460, 103–107 (2009).
Cui, G. et al. IL-7-induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161, 750–761 (2015).
Cong, J. et al. Dysfunction of natural killer cells by FBP1-induced inhibition of glycolysis during lung cancer progression. Cell. Metab. 28, 243–255 (2018). e245.
Cichocki, F. et al. ARID5B regulates metabolic programming in human adaptive NK cells. J. Exp. Med. 215, 2379–2395 (2018).
Orange, J. S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 132, 515–526 (2013).
Bjorkstrom, N. K. et al. Rapid expansion and long-term persistence of elevated NK cell numbers in humans infected with hantavirus. J. Exp. Med. 208, 13–21 (2011).
Beziat, V. et al. CMV drives clonal expansion of NKG2C+NK cells expressing self-specific KIRs in chronic hepatitis patients. Eur. J. Immunol. 42, 447–457 (2012).
Ram, D. R. et al. Tracking KLRC2 (NKG2C)+memory-like NK cells in SIV+and rhCMV+rhesus macaques. PLoS. Pathog. 14, e1007104 (2018).
Martinez-Martin, N. et al. An unbiased screen for human cytomegalovirus identifies neuropilin-2 as a central viral receptor. Cell 174, 1158–1171 (2018). e1119.
Hammer, Q. et al. Peptide-specific recognition of human cytomegalovirus strains controls adaptive natural killer cells. Nat. Immunol. 19, 453–463 (2018).
Rolle, A. et al. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+NK cell expansion. J. Clin. Invest. 124, 5305–5316 (2014).
Zhang, T., Scott, J. M., Hwang, I. & Kim, S. Cutting edge: antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J. Immunol. 190, 1402–1406 (2013).
Lee, J. et al. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 42, 431–442 (2015).
Shah, S. V. et al. CMV primes functional alternative signaling in adaptive Δg NK cells but is subverted by lentivirus infection in rhesus macaques. Cell Rep. 25, 2766–2774 (2018). e2763.
Luetke-Eversloh, M. et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS. Pathog. 10, e1004441 (2014).
Li, T. et al. Respiratory influenza virus infection induces memory-like liver NK cells in mice. J. Immunol. 198, 1242–1252 (2017).
Dou, Y. et al. Influenza vaccine induces intracellular immune memory of human NK cells. PLoS. One. 10, e0121258 (2015).
Keppel, M. P., Yang, L. & Cooper, M. A. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J. Immunol. 190, 4754–4762 (2013).
Ni, J. et al. Adoptively transferred natural killer cells maintain long-term antitumor activity by epigenetic imprinting and CD4(+) T cell help. Oncoimmunology 5, e1219009 (2016).
Romee, R. et al. Cytokine activation induces human memory-like NK cells. Blood 120, 4751–4760 (2012).
Sojka D. K., et al. Cutting edge: local proliferation of uterine tissue-resident NK cells during decidualization in mice. J. Immunol. 201, 2551–2556 (2018).
Fu, B. et al. Natural killer cells promote fetal development through the secretion of growth-promoting factors. Immunity 47, 1100–1113 (2017). e1106.
Gamliel, M. et al. Trained memory of human uterine NK cells enhances their function in subsequent pregnancies. Immunity 48, 951–962 (2018). e955.
Ni, J., Miller, M., Stojanovic, A., Garbi, N. & Cerwenka, A. Sustained effector function of IL-12/15/18–preactivated NK cells against established tumors. J. Exp. Med. 209, 2351–2365 (2012).
Romee, R. et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci. Transl. Med. 8, 357ra123–357ra123 (2016).
Vosshenrich, C. A. et al. A thymic pathway of mouse natural killer cell development characterized by expression of GATA-3 and CD127. Nat. Immunol. 7, 1217–1224 (2006).
Luther, C., Warner, K. & Takei, F. Unique progenitors in mouse lymph node develop into CD127+NK cells: thymus-dependent and thymus-independent pathways. Blood 117, 4012–4021 (2011).
Spits, H. & Di Santo, J. P. The expanding family of innate lymphoid cells: regulators and effectors of immunity and tissue remodeling. Nat. Immunol. 12, 21–27 (2011).
Mackley, E. C. et al. CCR7-dependent trafficking of RORgamma(+) ILCs creates a unique microenvironment within mucosal draining lymph nodes. Nat. Commun. 6, 5862 (2015).
Robinette, M. L. et al. IL-15 sustains IL-7R-independent ILC2 and ILC3 development. Nat. Commun. 8, 14601 (2017).
Knolle, P. A. & Wohlleber, D. Immunological functions of liver sinusoidal endothelial cells. Cell. Mol. Immunol. 13, 347 (2016).
Marra, F. & Tacke, F. Roles for chemokines in liver disease. Gastroenterology 147, 577–594 (2014). e571.
Liang, B. et al. Role of hepatocyte-derived IL-7 in maintenance of intrahepatic NKT cells and T cells and development of B cells in fetal liver. J. Immunol. 189, 4444–4450 (2012).
Sawa, Y. et al. Hepatic interleukin-7 expression regulates T cell responses. Immunity 30, 447–457 (2009).
Kaplan, D. H., Igyarto, B. Z. & Gaspari, A. A. Early immune events in the induction of allergic contact dermatitis. Nat. Rev. Immunol. 12, 114–124 (2012).
Carbone, T. et al. CD56highCD16-CD62L- NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J. Immunol. 184, 1102–1110 (2010).
Mazo, I. B. et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+T cells. Immunity 22, 259–270 (2005).
Taniguchi, H., Toyoshima, T., Fukao, K. & Nakauchi, H. Presence of hematopoietic stem cells in the adult liver. Nat. Med. 2, 198 (1996).
Kotton, D. N., Fabian, A. J. & Mulligan, R. C. A novel stem-cell population in adult liver with potent hematopoietic-reconstitution activity. Blood 106, 1574–1580 (2005).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 1, 426–432 (2000).
Kaech, S. M. et al. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4, 1191–1198 (2003).
Kondrack, R. M. et al. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 198, 1797–1806 (2003).
Zhang, X., Sun, S., Hwang, I., Tough, D. F. & Sprent, J. Potent and selective stimulation of memory-phenotype CD8+T cells in vivo by IL-15. Immunity 8, 591–599 (1998).
Becker, T. C. et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T Cells. J. Exp. Med. 195, 1541–1548 (2002).
Purton, J. F. et al. Antiviral CD4+memory T cells are IL-15 dependent. J. Exp. Med. 204, 951–961 (2007).
Firth, M. A. et al. Nfil3-independent lineage maintenance and antiviral response of natural killer cells. J. Exp. Med. 210, 2981–2990 (2013).
Martinez-Gonzalez, I. et al. ILC2 memory: recollection of previous activation. Immunol. Rev. 283, 41–53 (2018).
Martinez-Gonzalez, I., Matha, L., Steer, C. A. & Takei, F. Immunological memory of group 2 innate Lymphoid Cells. Trends Immunol. 38, 423–431 (2017).
Jing, X. et al. The formation of memory-like innate lymphoid cells 2 in allergic asthma. J. Immunol. 198, 194.117–194.117 (2017).
Acknowledgements
This work was supported by the Natural Science Foundation of China (#81788101, 81761128013, 81571522, 91642105, 91542114, and 91542000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wang, X., Peng, H. & Tian, Z. Innate lymphoid cell memory. Cell Mol Immunol 16, 423–429 (2019). https://doi.org/10.1038/s41423-019-0212-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0212-6
This article is cited by
-
Novel Findings on the Development and Immunological Functions of Palatine Tonsils
Clinical Reviews in Allergy & Immunology (2025)
-
Exploring the global immune landscape of peripheral blood mononuclear cells in H5N6-infected patient with single-cell transcriptomics
BMC Medical Genomics (2023)
-
Modeling the disruption of respiratory disease clinical trials by non-pharmaceutical COVID-19 interventions
Nature Communications (2022)
-
Chimeric antigen receptor- and natural killer cell receptor-engineered innate killer cells in cancer immunotherapy
Cellular & Molecular Immunology (2021)
-
Dynamic regulation of innate lymphoid cells in the mucosal immune system
Cellular & Molecular Immunology (2021)