Abstract
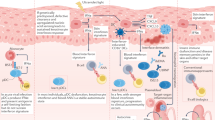
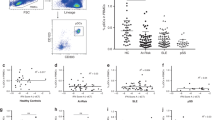
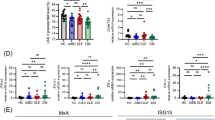
Systemic lupus erythematosus (SLE) is a complex, multiorgan autoimmune disorder. Although it is widely believed that SLE originates from immune cell dysregulation, the etiology of SLE is not yet clear. Here, we propose a new theory in which SLE can be directly initiated by molecular alterations in keratinocytes rather than immune cells. We found that the level of peroxisome proliferator-activated receptor gamma (PPARγ) is substantially reduced in the skin lesions of patients, and replicating this reduction in mice led to rapid disease onset with multiple hallmarks of SLE. As PPARγ decreases in keratinocytes, which is accompanied by increased occupancy of interferon regulatory factor 3 at the type I interferon locus, dendritic cells (DCs) are recruited to the epidermis and are activated by keratinocyte-secreted type I interferon. These activated DCs migrate to local draining lymph nodes, where they activate CD4+ T cells in a non-MHC II-dependent manner, promoting their differentiation into effector T cells and thus contributing to disease onset. Our study revealed that the dysregulation of keratinocytes can be a pathogenic driver of SLE and describes a new mouse model that mimics human SLE. Our data also emphasize the pivotal role of skin immunity in the onset of systemic autoimmune disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All data are available in the main text or the supplementary materials.
References
Tsokos GC. Autoimmunity and organ damage in systemic lupus erythematosus. Nat Immunol. 2020;21:605–14.
Tian J, Zhang D, Yao X, Huang Y, Lu Q. Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modeling study. Ann Rheum Dis. 2023;82:351–6.
Kobayashi T, Naik S, Nagao K. Choreographing Immunity in the Skin Epithelial Barrier. Immunity. 2019;50:552–65.
Kabashima K, Honda T, Ginhoux F, Egawa G. The immunological anatomy of the skin. Nat Rev Immunol. 2019;19:19–30.
Pasparakis M, Haase I, Nestle FO. Mechanisms regulating skin immunity and inflammation. Nat Rev Immunol. 2014;14:289–301.
Tsujihana K, Tanegashima K, Santo Y, Yamada H, Akazawa S, Nakao R, et al. Circadian protection against bacterial skin infection by epidermal CXCL14-mediated innate immunity. Proc Natl Acad Sci USA. 2022;119:e2116027119.
Stull C, Sprow G, Werth VP. Cutaneous Involvement in Systemic Lupus Erythematosus: A Review for the Rheumatologist. J Rheumatol. 2023;50:27–35.
Epstein JH, Tuffanelli D, Dubois EL. Light Sensitivity And Lupus Erythematosus. Arch Dermatol. 1965;91:483–5.
Sanders CJ, Van Weelden H, Kazzaz GA, Sigurdsson V, Toonstra J, Bruijnzeel-Koomen CA. Photosensitivity in patients with lupus erythematosus: a clinical and photobiological study of 100 patients using a prolonged phototest protocol. Br J Dermatol. 2003;149:131–7.
Hile GA, Coit P, Xu B, Victory AM, Gharaee-Kermani M, Estadt SN, et al. Regulation of Photosensitivity by the Hippo Pathway in Lupus Skin. Arthritis Rheumatol. 2023;75:1216–28.
Der E, Suryawanshi H, Morozov P, Kustagi M, Goilav B, Ranabothu S, et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat Immunol. 2019;20:915–27.
Psarras A, Alase A, Antanaviciute A, Carr IM, Md Yusof MY, Wittmann M, et al. Functionally impaired plasmacytoid dendritic cells and nonhaematopoietic sources of type I interferon characterize human autoimmunity. Nat Commun. 2020;11:6149.
Sarkar MK, Hile GA, Tsoi LC, Xing X, Liu J, Liang Y, et al. Photosensitivity and type I IFN responses in cutaneous lupus are driven by epidermal-derived interferon kappa. Ann Rheum Dis. 2018;77:1653–64.
Tsoi LC, Hile GA, Berthier CC, Sarkar MK, Reed TJ, Liu J, et al. Hypersensitive IFN Responses in Lupus Keratinocytes Reveal Key Mechanistic Determinants in Cutaneous Lupus. J Immunol. 2019;202:2121–30.
Billi AC, Ma F, Plazyo O, Gharaee-Kermani M, Wasikowski R, Hile GA, et al. Nonlesional lupus skin contributes to inflammatory education of myeloid cells and primes for cutaneous inflammation. Sci Transl Med. 2022;14:eabn2263.
Liu Y, Luo S, Zhan Y, Wang J, Zhao R, Li Y, et al. Increased Expression of PPAR-γ Modulates Monocytes Into a M2-Like Phenotype in SLE Patients: An Implicative Protective Mechanism and Potential Therapeutic Strategy of Systemic Lupus Erythematosus. Front Immunol. 2020;11:579372.
Zhao W, Berthier CC, Lewis EE, McCune WJ, Kretzler M, Kaplan MJ. The peroxisome-proliferator activated receptor-γ agonist pioglitazone modulates aberrant T-cell responses in systemic lupus erythematosus. Clin Immunol. 2013;149:119–32.
Hernandez-Quiles M, Broekema MF, Kalkhoven E. PPARgamma in Metabolism, Immunity, and Cancer: Unified and Diverse Mechanisms of Action. Front Endocrinol. 2021;12:624112.
Aprahamian TR, Bonegio RG, Weitzner Z, Gharakhanian R, Rifkin IR. Peroxisome proliferator-activated receptor gamma agonists in the prevention and treatment of murine systemic lupus erythematosus. Immunology. 2014;142:363–73.
Hasni S, Temesgen-Oyelakin Y, Davis M, Chu J, Poncio E, Naqi M, et al. Peroxisome proliferator activated receptor-γ agonist pioglitazone improves vascular and metabolic dysfunction in systemic lupus erythematosus. Ann Rheum Dis. 2022;81:1576–84.
Sahu RP, DaSilva SC, Rashid B, Martel KC, Jernigan D, Mehta SR, et al. Mice lacking epidermal PPARγ exhibit a marked augmentation in photocarcinogenesis associated with increased UVB-induced apoptosis, inflammation and barrier dysfunction. Int J Cancer. 2012;131:E1055–66.
Hanley K, Jiang Y, Crumrine D, Bass NM, Appel R, Elias PM, et al. Activators of the nuclear hormone receptors PPARalpha and FXR accelerate the development of the fetal epidermal permeability barrier. J Clin Invest. 1997;100:705–12.
Chong HC, Tan MJ, Philippe V, Tan SH, Tan CK, Ku CW, et al. Regulation of epithelial–mesenchymal IL-1 signaling by PPARbeta/delta is essential for skin homeostasis and wound healing. J Cell Biol. 2009;184:817–31.
Michalik L, Desvergne B, Tan NS, Basu-Modak S, Escher P, Rieusset J, et al. Impaired skin wound healing in peroxisome proliferator-activated receptor (PPAR)alpha and PPARbeta mutant mice. J Cell Biol. 2001;154:799–814.
Nelson WG, Sun TT. The 50- and 58-kdalton keratin classes as molecular markers for stratified squamous epithelia: cell culture studies. J Cell Biol. 1983;97:244–51.
Cottle DL, Ursino G, Jones LK, Tham MS, Zylberberg AK, Smyth IM. Topical Aminosalicylic Acid Improves Keratinocyte Differentiation in an Inducible Mouse Model of Harlequin Ichthyosis. Cell Rep Med. 2020;1:100129.
Yang M, Cao P, Zhao Z, Wang Z, Jia C, Guo Y, et al. An Enhanced Expression Level of CXCR3 on Tfh-like Cells from Lupus Skin Lesions Rather Than Lupus Peripheral Blood. Clin Immunol. 2021;226:108717.
Hanaoka H, Nishimoto T, Okazaki Y, Takeuchi T, Kuwana M. A unique thymus-derived regulatory T-cell subset associated with systemic lupus erythematosus. Arthritis Res Ther. 2020;22:88.
Patel J, Vazquez T, Chin F, Keyes E, Yan D, Diaz D, et al. Multidimensional Immune Profiling of Cutaneous Lupus Erythematosus In Vivo Stratified by Patient Response to Antimalarials. Arthritis Rheumatol. 2022;74:1687–98.
Ben-Sasson SZ, Hu-Li J, Quiel J, Cauchetaux S, Ratner M, Shapira I, et al. IL-1 acts directly on CD4 T cells to enhance their antigen-driven expansion and differentiation. Proc Natl Acad Sci USA. 2009;106:7119–24.
Muth S, Schütze K, Schild H, Probst HC. Release of dendritic cells from cognate CD4+ T-cell recognition results in impaired peripheral tolerance and fatal cytotoxic T-cell mediated autoimmunity. Proc Natl Acad Sci USA. 2012;109:9059–64.
Azukizawa H, Sano S, Kosaka H, Sumikawa Y, Itami S. Prevention of toxic epidermal necrolysis by regulatory T cells. Eur J Immunol. 2005;35:1722–30.
Crow MK. Pathogenesis of systemic lupus erythematosus: risks, mechanisms and therapeutic targets. Ann Rheum Dis. 2023;82:999–1014.
Barker JN, Mitra RS, Griffiths CE, Dixit VM, Nickoloff BJ. Keratinocytes as initiators of inflammation. Lancet. 1991;337:211–4.
Gaipl US, Kuhn A, Sheriff A, Munoz LE, Franz S, Voll RE, et al. Clearance of apoptotic cells in human SLE. Curr Dir Autoimmun. 2006;9:173–87.
Kuhn A, Herrmann M, Kleber S, Beckmann-Welle M, Fehsel K, Martin-Villalba A, et al. Accumulation of apoptotic cells in the epidermis of patients with cutaneous lupus erythematosus after ultraviolet irradiation. Arthritis Rheum. 2006;54:939–50.
Scholtissek B, Zahn S, Maier J, Klaeschen S, Braegelmann C, Hoelzel M, et al. Immunostimulatory Endogenous Nucleic Acids Drive the Lesional Inflammation in Cutaneous Lupus Erythematosus. J Invest Dermatol. 2017;137:1484–92.
Golan DT, Borel Y. Increased photosensitivity to near-ultraviolet light in murine SLE. J Immunol. 1984;132:705–10.
Mondini M, Vidali M, Airò P, De Andrea M, Riboldi P, Meroni PL, et al. Role of the interferon-inducible gene IFI16 in the etiopathogenesis of systemic autoimmune disorders. Ann N Y Acad Sci. 2007;1110:47–56.
Skopelja-Gardner S, An J, Tai J, Tanaka L, Sun X, Hermanson P, et al. The early local and systemic Type I interferon responses to ultraviolet B light exposure are cGAS dependent. Sci Rep. 2020;10:7908.
Skopelja-Gardner, S, Tai J, Sun X, Tanaka L, Kuchenbecker JA, Snyder JM, et al. Acute skin exposure to ultraviolet light triggers neutrophil-mediated kidney inflammation. Proc Natl Acad Sci USA. 2021;118:e2019097118.
Kolios AGA, Tsokos GC. Skin-kidney crosstalk in SLE. Nat Rev Rheumatol. 2021;17:253–4.
Stannard JN, Kahlenberg JM. Cutaneous lupus erythematosus: updates on pathogenesis and associations with systemic lupus. Curr Opin Rheumatol. 2016;28:453–9.
Mori S, Kohyama M, Yasumizu Y, Tada A, Tanzawa K, Shishido T, et al. Neoself-antigens are the primary target for autoreactive T cells in human lupus. Cell. 2024;187:6071–87.
Greb JE, Goldminz AM, Elder JT, Lebwohl MG, Gladman DD, Wu JJ, et al. Psoriasis. Nat Rev Dis Prim. 2016;2:16082.
Williams HC, Burney PG, Pembroke AC, Hay RJ. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131:406–16.
Tian J, Zhang D, Kurbatov V, Wang Q, Wang Y, Fang D, et al. 5-Fluorouracil efficacy requires anti-tumor immunity triggered by cancer-cell-intrinsic STING. EMBO J. 2021;40:e106065.
Khsheibun R, Paperna T, Volkowich A, Lejbkowicz I, Avidan N, Miller A. Gene expression profiling of the response to interferon beta in Epstein‒Barr-transformed and primary B cells of patients with multiple sclerosis. PLoS One. 2014;9:e102331.
Zheng M, Hu Z, Mei X, Ouyang L, Song Y, Zhou W, et al. Single-cell sequencing shows cellular heterogeneity of cutaneous lesions in lupus erythematosus. Nat Commun. 2022;13:7489.
Funding
The CAMS Innovation Fund for Medical Sciences grant No. 2021-I2M-1-059. Nonprofit Central Research Institute Fund of the Chinese Academy of Medical Sciences grant Nos. 2021-RC320-001 and 2020-RC320-003. National Natural Science Foundation of China grant No. 81830097 and No. 82203933. the Special Program of the National Natural Science Foundation of China grant No. 32141004.
Author information
Authors and Affiliations
Contributions
Conceptualization: JT, QL. Methodology: JT, DZ. Visualization: JT, LS, DZ. Funding acquisition: QL. Project administration: QL. Supervision: JT, QL. Writing – original draft: JT, LS. Writing – review & editing: LS, XY, JL, MZ, SK, and DY.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests. QL and DY are editorial board members of Cellular & Molecular Immunology, but they have not been involved in the peer review or the decision-making of the article.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tian, J., Shi, L., Zhang, D. et al. Dysregulation in keratinocytes drives systemic lupus erythematosus onset. Cell Mol Immunol 22, 83–96 (2025). https://doi.org/10.1038/s41423-024-01240-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-024-01240-z
Keywords
This article is cited by
-
Increasing evidence for the pathogenic role of keratinocytes in lupus
Cellular & Molecular Immunology (2025)