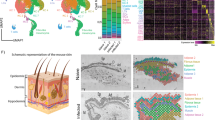
Abstract
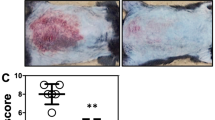
The immune response of the skin to danger signals involves rapid recruitment of neutrophils, but their excessive accumulation leads to inflammatory skin diseases, such as psoriasis; however, the mechanisms governing their initiation and resolution are poorly understood. Here, we revealed a dynamic immunoregulatory role of dermal white adipose tissue (dWAT) in the progression and resolution of neutrophilic skin inflammation in an imiquimod-induced psoriasis mouse model. During inflammation onset, dWAT repopulates PDGFRA+ preadipocytes (pAds), which secrete CXCL1 and SAA3, attracting and activating CXCR2+ neutrophils. These neutrophils further activate pAds through the IL-1R-NFκB-C/EBPδ pathway, establishing a self-sustaining inflammatory loop. Paradoxically, prolonged IL-1β signaling triggers PPARγ-dependent adipogenesis, transitioning pAds into anti-inflammatory early adipocytes that resolve neutrophilic inflammation via lipid mediators. Inhibition of adipogenesis, via pharmacological or genetic inhibition of PPARγ, disrupts the formation of early adipocytes, prevents neutrophil regression, and exacerbates inflammation. Analysis of human psoriatic cells revealed a C/EBPδ+ dermal fibroblast (dFB) subpopulation enriched with preadipocytes, the IL-1 pathway, and inflammatory gene signatures. Furthermore, transcriptomic analyses revealed a negative correlation between the neutrophil-related inflammatory response and the dermal lipogenesis response in generalized pustular psoriasis. Together, our findings reveal the dual role of dWAT: PDGFRA+ pAds initiate inflammation via CXCL1/IL-1β crosstalk with neutrophils, whereas PPARγ-driven adipogenesis resolves this process through lipid mediators. This work establishes dWAT as a critical immunomodulatory hub and proposes adipogenic reprogramming of proinflammatory fibroblasts or topical delivery of early adipocyte lipids as innovative therapies for neutrophil-driven skin diseases, such as psoriasis and ulcers.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Peiseler M, Kubes P. More friend than foe: the emerging role of neutrophils in tissue repair. J Clin Invest. 2019;129:2629–39. https://doi.org/10.1172/JCI124616.
Qu J, Jin J, Zhang M, Ng LG. Neutrophil diversity and plasticity: Implications for organ transplantation. Cell Mol Immunol. 2023;20:993–1001. https://doi.org/10.1038/s41423-023-01058-1.
Oliveira-Costa KM, Menezes GB, Paula Neto HA. Neutrophil accumulation within tissues: a damage x healing dichotomy. Biomed Pharmacother. 2022;145:112422 https://doi.org/10.1016/j.biopha.2021.112422.
Marzano AV, Ortega-Loayza AG, Heath M, Morse D, Genovese G, Cugno M. Mechanisms of inflammation in neutrophil-mediated skin diseases. Front Immunol. 2019;10:1059 https://doi.org/10.3389/fimmu.2019.01059.
Chiang CC, Cheng WJ, Korinek M, Lin CY, Hwang TL. Neutrophils in Psoriasis. Front Immunol. 2019;10:2376 https://doi.org/10.3389/fimmu.2019.02376.
Schon MP, Broekaert SM, Erpenbeck L. Sexy again: the renaissance of neutrophils in psoriasis. Exp Dermatol. 2017;26:305–11. https://doi.org/10.1111/exd.13067.
Mocsai A. Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J Exp Med. 2013;210:1283–99. https://doi.org/10.1084/jem.20122220.
Pinegin B, Vorobjeva N, Pinegin V. Neutrophil extracellular traps and their role in the development of chronic inflammation and autoimmunity. Autoimmun Rev. 2015;14:633–40. https://doi.org/10.1016/j.autrev.2015.03.002.
Young KZ, Sarkar MK, Gudjonsson JE. Pathophysiology of generalized pustular psoriasis. Exp Dermatol. 2023;32:1194–203. https://doi.org/10.1111/exd.14768.
Samotij D, Szczech J, Reich A. Generalized Pustular Psoriasis: Divergence of Innate and Adaptive Immunity. Int J Mol Sci. 2021;22. https://doi.org/10.3390/ijms22169048
Ikeda S, Takahashi H, Suga Y, Eto H, Etoh T, Okuma K, et al. Therapeutic depletion of myeloid lineage leukocytes in patients with generalized pustular psoriasis indicates a major role for neutrophils in the immunopathogenesis of psoriasis. J Am Acad Dermatol. 2013;68:609–17. https://doi.org/10.1016/j.jaad.2012.09.037.
Lambert S, Hambro CA, Johnston A, Stuart PE, Tsoi LC, Nair RP, Elder JT. Neutrophil extracellular traps induce human Th17 cells: effect of psoriasis-associated TRAF3IP2 genotype. J Invest Dermatol. 2019;139:1245–53. https://doi.org/10.1016/j.jid.2018.11.021.
Shao S, Fang H, Zhang J, Jiang M, Xue K, Ma J, et al. Neutrophil exosomes enhance the skin autoinflammation in generalized pustular psoriasis by activating keratinocytes. FASEB J. 2019;33:6813–28. https://doi.org/10.1096/fj.201802090RR.
Chen J, Zhu Z, Li Q, Lin Y, Dang E, Meng H, et al. Neutrophils enhance cutaneous vascular dilation and permeability to aggravate psoriasis by releasing matrix metallopeptidase 9. J Invest Dermatol. 2021;141:787–99. https://doi.org/10.1016/j.jid.2020.07.028.
Sun L, Zhang X, Wu S, Liu Y, Guerrero-Juarez CF, Liu W, et al. Dynamic interplay between IL-1 and WNT pathways in regulating dermal adipocyte lineage cells during skin development and wound regeneration. Cell Rep. 2023;42:112647 https://doi.org/10.1016/j.celrep.2023.112647.
Zhang, LJ, Guerrero-Juarez CF, Chen SX, Zhang X, Yin M, Li F, et al. Diet-induced obesity promotes infection by impairment of the innate antimicrobial defense function of dermal adipocyte progenitors. Sci Transl Med. 2021;13. https://doi.org/10.1126/scitranslmed.abb5280
Zhang Z, Shao M, Hepler C, Zi Z, Zhao S, An YA, et al. Dermal adipose tissue has high plasticity and undergoes reversible dedifferentiation in mice. J Clin Investig. 2019;129:5327–42.
Zhang LJ, Chen SX, Guerrero-Juarez CF, Li F, Tong Y, Liang Y, et al. Age-related loss of innate immune antimicrobial function of dermal fat is mediated by transforming growth factor beta. Immunity. 2019;50:121–136 e125. https://doi.org/10.1016/j.immuni.2018.11.003.
Zhang LJ, Guerrero-Juarez CF, Hata T, Bapat SP, Ramos R, Plikus MV, Gallo RL. Dermal adipocytes protect against invasive Staphylococcus aureus skin infection. Science. 2015;347:67–71. https://doi.org/10.1126/science.1260972.
Chen SX, Zhang LJ, Gallo RL. Dermal white adipose tissue: a newly recognized layer of skin innate defense. J Invest Dermatol. 2019;139:1002–9. https://doi.org/10.1016/j.jid.2018.12.031.
Sanda GE, Belur AD, Teague HL, Mehta NN. Emerging associations between neutrophils, atherosclerosis, and psoriasis. Curr Atheroscler Rep. 2017;19:53 https://doi.org/10.1007/s11883-017-0692-8.
Zhu Z, Chen J, Lin Y, Zhang C, Li W, Qiao H, et al. Aryl hydrocarbon receptor in cutaneous vascular endothelial cells restricts psoriasis development by negatively regulating neutrophil recruitment. J Invest Dermatol. 2020;140:1233–1243 e1239. https://doi.org/10.1016/j.jid.2019.11.022.
Gangwar RS, Gudjonsson JE, Ward NL. Mouse models of psoriasis: a comprehensive review. J Invest Dermatol. 2022;142:884–97. https://doi.org/10.1016/j.jid.2021.06.019.
Hoffmann K, Sperling K, Olins AL, Olins DE. The granulocyte nucleus and lamin B receptor: avoiding the ovoid. Chromosoma. 2007;116:227–35. https://doi.org/10.1007/s00412-007-0094-8.
Rose S, Misharin A, Perlman H. A novel Ly6C/Ly6G-based strategy to analyze the mouse splenic myeloid compartment. Cytometry. 2012;81:343–50. https://doi.org/10.1002/cyto.a.22012.
Lee PY, Wang JX, Parisini E, Dascher CC, Nigrovic PA. Ly6 family proteins in neutrophil biology. J Leukoc Biol. 2013;94:585–94. https://doi.org/10.1189/jlb.0113014.
Asp M, Giacomello S, Larsson L, Wu C, Fürth D, Qian X, et al. A Spatiotemporal organ-wide gene expression and cell atlas of the developing human heart. Cell. 2019;179:1647–1660.e1619. https://doi.org/10.1016/j.cell.2019.11.025.
Ntambi JM, Young-Cheul K. Adipocyte differentiation and gene expression. J Nutr. 2000;130:3122S–3126S. https://doi.org/10.1093/jn/130.12.3122S.
Hallenborg P, Feddersen S, Francoz S, Murano I, Sundekilde U, Petersen RK, et al. Mdm2 controls CREB-dependent transactivation and initiation of adipocyte differentiation. Cell Death Differ. 2012;19:1381–9. https://doi.org/10.1038/cdd.2012.15.
Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–73. https://doi.org/10.1016/j.cmet.2006.07.001.
Sawant KV, Sepuru KM, Penaranda B, Lowry E, Garofalo RP, Rajarathnam K. Chemokine Cxcl1-Cxcl2 heterodimer is a potent neutrophil chemoattractant. J Leukoc Biol. 2023;114:666–71. https://doi.org/10.1093/jleuko/qiad097.
Sawant KV, Poluri KM, Dutta AK, Sepuru KM, Troshkina A, Garofalo RP, Rajarathnam K. Chemokine CXCL1 mediated neutrophil recruitment: Role of glycosaminoglycan interactions. Sci Rep. 2016;6:33123. https://doi.org/10.1038/srep33123.
Lefterova MI, Haakonsson AK, Lazar MA, Mandrup S. PPARgamma and the global map of adipogenesis and beyond. Trends Endocrinol Metab. 2014;25:293–302. https://doi.org/10.1016/j.tem.2014.04.001.
Sun W, Dong H, Balaz M, Slyper M, Drokhlyansky E, Colleluori G, et al. snRNA-seq reveals a subpopulation of adipocytes that regulates thermogenesis. Nature. 2020;587:98–102. https://doi.org/10.1038/s41586-020-2856-x.
Festa E, Fretz J, Berry R, Schmidt B, Rodeheffer M, Horowitz M, Horsley V. Adipocyte lineage cells contribute to the skin stem cell niche to drive hair cycling. Cell. 2011;146:761–71. https://doi.org/10.1016/j.cell.2011.07.019.
Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the hematopoietic microenvironment. Nature. 2009;460:259–63. https://doi.org/10.1038/nature08099.
Wang J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018;371:531–9. https://doi.org/10.1007/s00441-017-2785-7.
Suleimanov, SK, Efremov YM, Klyucherev TO, Salimov EL, Ragimov AA, Timashev PS, Vlasova II. Radical-Generating Activity, Phagocytosis, and Mechanical Properties of Four Phenotypes of Human Macrophages. Int J Mol Sci. 2024;25. https://doi.org/10.3390/ijms25031860
Strizova Z, Benesova I, Bartolini R, Novysedlak R, Cecrdlova E, Foley LK, Striz I. M1/M2 macrophages and their overlaps - myth or reality?. Clin Sci. 2023;137:1067–93. https://doi.org/10.1042/CS20220531.
Li YH, Zhang Y, Pan G, Xiang LX, Luo DC, Shao JZ. Occurrences and functions of Ly6C(hi) and Ly6C(lo) macrophages in health and disease. Front Immunol. 2022;13:901672 https://doi.org/10.3389/fimmu.2022.901672.
Zhang C, Duan J, Huang Y, Chen M. Enhanced skin delivery of therapeutic peptides using spicule-based topical delivery systems. Pharmaceutics. 2021:13. https://doi.org/10.3390/pharmaceutics13122119
Zhang S, Ou H, Liu C, Zhang Y, Mitragotri S, Wang D, Chen M. Skin delivery of hydrophilic biomacromolecules using marine sponge spicules. Mol Pharm. 2017;14:3188–3200. https://doi.org/10.1021/acs.molpharmaceut.7b00468.
Reynolds, G, Vegh P, Fletcher J, Poyner E, Stephenson E, Goh I, et al. Developmental cell programs are co-opted in inflammatory skin disease. Science 2021;371. https://doi.org/10.1126/science.aba6500
Russell-Goldman E, Murphy GF. The pathobiology of skin aging: new insights into an old dilemma. Am J Pathol. 2020;190:1356–69. https://doi.org/10.1016/j.ajpath.2020.03.007.
Chen Q, Shou P, Zheng C, Jiang M, Cao G, Yang Q, et al. Fate decision of mesenchymal stem cells: adipocytes or osteoblasts?. Cell Death Differ. 2016;23:1128–39. https://doi.org/10.1038/cdd.2015.168.
Kesharwani D, Brown AC. Navigating the adipocyte precursor niche: cell‒cell interactions, regulatory mechanisms and implications for adipose tissue homeostasis. J Cell Signal. 2024;5:65–86. https://doi.org/10.33696/signaling.5.114.
Jiang Y, Berry DC, Jo A, Tang W, Arpke RW, Kyba M, Graff JM. A PPARgamma transcriptional cascade directs adipose progenitor cell-niche interaction and niche expansion. Nat Commun. 2017;8:15926. https://doi.org/10.1038/ncomms15926.
Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140:3939–49. https://doi.org/10.1242/dev.080549.
Johnston A, Xing X, Wolterink L, Barnes DH, Yin Z, Reingold L, et al. IL-1 and IL-36 are dominant cytokines in generalized pustular psoriasis. J Allergy Clin Immunol. 2017;140:109–20. https://doi.org/10.1016/j.jaci.2016.08.056.
Schmidt BA, Horsley V. Intradermal adipocytes mediate fibroblast recruitment during skin wound healing. Development. 2013;140:1517–27. https://doi.org/10.1242/dev.087593.
Cordier-Dirikoc S, Pedretti N, Garnier J, Clarhaut-Charreau S, Ryffel B, Morel F, et al. Dermal fibroblasts are the key sensors of aseptic skin inflammation through interleukin 1 release by lesioned keratinocytes. Front Immunol. 2022;13:984045 https://doi.org/10.3389/fimmu.2022.984045.
Boothby IC, Kinet MJ, Boda DP, Kwan EY, Clancy S, Cohen JN, et al. Early-life inflammation primes a T helper 2 cell-fibroblast niche in skin. Nature. 2021;599:667–72. https://doi.org/10.1038/s41586-021-04044-7.
Zdanowska N, Kasprowicz-Furmanczyk M, Placek W, Owczarczyk-Saczonek A. The role of chemokines in psoriasis-an overview. Medicina. 2021;57. https://doi.org/10.3390/medicina57080754
Zeng J, Chen X, Lei K, Wang D, Lin L, Wang Y, et al. Mannan-binding lectin promotes keratinocyte to produce CXCL1 and enhances neutrophil infiltration at the early stages of psoriasis. Exp Dermatol. 2019;28:1017–24. https://doi.org/10.1111/exd.13995.
Gillitzer R, Ritter U, Spandau U, Goebeler M, Brocker EB. Differential expression of GRO-alpha and IL-8 mRNA in psoriasis: a model for neutrophil migration and accumulation in vivo. J Invest Dermatol. 1996;107:778–82. https://doi.org/10.1111/1523-1747.ep12371803.
De Buck M, Gouwy M, Wang JM, Van Snick J, Proost P, Struyf S, Van Damme J. The cytokine-serum amyloid A-chemokine network. Cytokine Growth Factor Rev. 2016;30:55–69. https://doi.org/10.1016/j.cytogfr.2015.12.010.
Uhlar CM, Whitehead AS. Serum amyloid A, the major vertebrate acute-phase reactant. Eur J Biochem. 1999;265:501–23. https://doi.org/10.1046/j.1432-1327.1999.00657.x.
Meek RL, Benditt EP. Amyloid A gene family expression in different mouse tissues. J Exp Med. 1986;164:2006–17. https://doi.org/10.1084/jem.164.6.2006.
Bing Z, Huang JH, Liao WS. NFkappa B interacts with serum amyloid A3 enhancer factor to synergistically activate mouse serum amyloid A3 gene transcription. J Biol Chem. 2000;275:31616–23. https://doi.org/10.1074/jbc.M005378200.
Huang JH, Liao WS. Synergistic induction of mouse serum amyloid A3 promoter by the inflammatory mediators IL-1 and IL-6. J Interferon Cytokine Res. 1999;19:1403–11. https://doi.org/10.1089/107999099312867.
Shimizu H, Yamamoto K. NF-kappa B and C/EBP transcription factor families synergistically function in mouse serum amyloid A gene expression induced by inflammatory cytokines. Gene. 1994;149:305–10. https://doi.org/10.1016/0378-1119(94)90166-x.
Eklund KK, Niemi K, Kovanen PT. Immune functions of serum amyloid A. Crit Rev Immunol. 2012;32:335–48. https://doi.org/10.1615/critrevimmunol.v32.i4.40.
den Hartigh LJ, Wang S, Goodspeed L, Ding Y, Averill M, Subramanian S, et al. Deletion of serum amyloid A3 improves high fat high sucrose diet-induced adipose tissue inflammation and hyperlipidemia in female mice. PLoS One. 2014;9:e108564 https://doi.org/10.1371/journal.pone.0108564.
Lin Y, Rajala MW, Berger JP, Moller DE, Barzilai N, Scherer PE. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J Biol Chem. 2001;276:42077–83. https://doi.org/10.1074/jbc.M107101200.
Bai S, Zhang Z, Hou S, Liu X. Influence of different types of contact hypersensitivity on imiquimod-induced psoriasis-like inflammation in mice. Mol Med Rep. 2016;14:671–80. https://doi.org/10.3892/mmr.2016.5299.
Wang X, Sun J, Hu J. IMQ Induced K14-VEGF mouse: a stable and long-term mouse model of psoriasis-like inflammation. PLoS One. 2015;10:e0145498 https://doi.org/10.1371/journal.pone.0145498.
Kanamori Y, Murakami M, Sugiyama M, Hashimoto O, Matsui T, Funaba M. Interleukin-1beta (IL-1beta) transcriptionally activates hepcidin by inducing CCAAT enhancer-binding protein delta (C/EBPdelta) expression in hepatocytes. J Biol Chem. 2017;292:10275–87. https://doi.org/10.1074/jbc.M116.770974.
Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, et al. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–43. https://doi.org/10.1038/ni.1721.
Svotelis A, Doyon G, Bernatchez G, Désilets A, Rivard N, Asselin C. IL-1β-dependent regulation of C/EBPδ transcriptional activity. Biochem Biophys Res Commun. 2005;328:461–70. https://doi.org/10.1016/j.bbrc.2005.01.002.
Hofwimmer K, de Paula Souza J, Subramanian N, Vujičić M, Rachid L, Méreau H, et al. IL-1beta promotes adipogenesis by directly targeting adipocyte precursors. Nat Commun. 2024;15:7957. https://doi.org/10.1038/s41467-024-51938-x.
Croasdell A, Duffney PF, Kim N, Lacy SH, Sime PJ, Phipps RP. PPARgamma and the innate immune system mediate the resolution of inflammation. PPAR Res. 2015;2015:549691 https://doi.org/10.1155/2015/549691.
Qadri M, Jay GD, Zhang LX, Schmidt TA, Totonchy J, Elsaid KA. Proteoglycan-4 is an essential regulator of synovial macrophage polarization and inflammatory macrophage joint infiltration. Arthritis Res Ther. 2021;23:241 https://doi.org/10.1186/s13075-021-02621-9.
Das N, Schmidt TA, Krawetz RJ, Dufour A. Proteoglycan 4: From Mere lubricant to regulator of tissue homeostasis and inflammation: does proteoglycan 4 have the ability to buffer the inflammatory response?. Bioessays. 2019;41:e1800166. https://doi.org/10.1002/bies.201800166.
Alquraini A, Garguilo S, D'Souza G, Zhang LX, Schmidt TA, Jay GD, Elsaid KA. The interaction of lubricin/proteoglycan 4 (PRG4) with toll-like receptors 2 and 4: an anti-inflammatory role of PRG4 in synovial fluid. Arthritis Res Ther. 2015;17:353 https://doi.org/10.1186/s13075-015-0877-x.
Tang W, Zeve D, Suh JM, Bosnakovski D, Kyba M, Hammer RE, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–6. https://doi.org/10.1126/science.1156232.
Ma F, Plazyo O, Billi AC, Tsoi LC, Xing X, Wasikowski R, et al. Single cell and spatial sequencing define processes by which keratinocytes and fibroblasts amplify inflammatory responses in psoriasis. Nat Commun. 2023;14:3455. https://doi.org/10.1038/s41467-023-39020-4.
Yang Loureiro Z, Joyce S, DeSouza T, Solivan-Rivera J, Desai A, Skritakis P, et al. Wnt signaling preserves progenitor cell multipotency during adipose tissue development. Nat Metab. 2023;5:1014–28. https://doi.org/10.1038/s42255-023-00813-y.
Ehrlund A, Mejhert N, Lorente-Cebrián S, Aström G, Dahlman I, Laurencikiene J, Rydén M. Characterization of the Wnt inhibitors secreted frizzled-related proteins (SFRPs) in human adipose tissue. J Clin Endocrinol Metab. 2013;98:E503–508. https://doi.org/10.1210/jc.2012-3416.
Hu E, Zhu Y, Fredrickson T, Barnes M, Kelsell D, Beeley L, Brooks D. Tissue restricted expression of two human Frzbs in preadipocytes and pancreas. Biochem Biophys Res Commun. 1998;247:287–93. https://doi.org/10.1006/bbrc.1998.8784.
Curcic S, Holzer M, Frei R, Pasterk L, Schicho R, Heinemann A, Marsche G. Neutrophil effector responses are suppressed by secretory phospholipase A2 modified HDL. Biochim Biophys Acta. 2015;1851:184–93. https://doi.org/10.1016/j.bbalip.2014.11.010.
Lin P, Welch EJ, Gao XP, Malik AB, Ye RD. Lysophosphatidylcholine modulates neutrophil oxidant production through elevation of cyclic AMP. J Immunol. 2005;174:2981–9. https://doi.org/10.4049/jimmunol.174.5.2981.
Peter C, Waibel M, Radu CG, Yang LV, Witte ON, Schulze-Osthoff K, et al. Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J Biol Chem. 2008;283:5296–305. https://doi.org/10.1074/jbc.M706586200.
Frasch SC, Berry KZ, Fernandez-Boyanapalli R, Jin HS, Leslie C, Henson PM, et al. NADPH oxidase-dependent generation of lysophosphatidylserine enhances clearance of activated and dying neutrophils via G2A. J Biol Chem. 2008;283:33736–49. https://doi.org/10.1074/jbc.M807047200.
Castoldi A, Monteiro LB, van Teijlingen Bakker N, Sanin DE, Rana N, Corrado M, et al. Triacylglycerol synthesis enhances macrophage inflammatory function. Nat Commun. 2020;11:4107. https://doi.org/10.1038/s41467-020-17881-3.
Mottillo EP, Balasubramanian P, Lee YH, Weng C, Kershaw EE, Granneman JG. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic beta3-adrenergic receptor activation. J Lipid Res. 2014;55:2276–86. https://doi.org/10.1194/jlr.M050005.
Liao, Y, Sun L, Nie M, Li J, Huang X, Heng S, et al. Modulation of skin inflammatory responses by aluminum adjuvant. Pharmaceutics. 2023;15. https://doi.org/10.3390/pharmaceutics15020576
Neu, SD, Strzepa A, Martin D, Sorci-Thomas MG, Pritchard KA J.r, Dittel BN. Myeloperoxidase Inhibition Ameliorates Plaque Psoriasis in Mice. Antioxidants. 2021;10. https://doi.org/10.3390/antiox10091338
O’Brien CE, Bonanno L, Zhang H, Wyss-Coray T. Beclin 1 regulates neuronal transforming growth factor-beta signaling by mediating recycling of the type I receptor ALK5. Mol Neurodegener. 2015;10:69 https://doi.org/10.1186/s13024-015-0065-0.
Majzoub RN, Chan CL, Ewert KK, Silva BF, Liang KS, Safinya CR. Fluorescence microscopy colocalization of lipid-nucleic acid nanoparticles with wildtype and mutant Rab5-GFP: a platform for investigating early endosomal events. Biochim Biophys Acta. 2015;1848:1308–18. https://doi.org/10.1016/j.bbamem.2015.03.001.
Acknowledgements
L-JZ is supported by the National Key R&D Program of China (2023YFC2508102), the National Natural Science Foundation of China (82373879), the Fujian Provincial Natural Science Foundation of China (2024J011009), and the Xiamen University Double First Class Construction Project (Biology) (DFC2024004). R. W. is supported by the Yunnan University Medical Research Foundation (YDYXJJ2024-0022) and Yunnan Provincial Department of Education Scientific Research Fund Project (2025J0020). We thank Dr. Jiahuai Han from Xiamen University for providing the IL1r1-/- mice and Dr. Yuling Shi from Fudan University for providing the Pparg-floxed mice. We thank the flow cytometry and confocal microscopic core facility at Xiamen University for flow cytometry, sorting and imaging studies.
Author information
Authors and Affiliations
Contributions
Conceptualization, TX, WZ, RW, MC, and LZ; methodology, TX, WZ, RW, XZ, RX, CZ, WL, and LZ; investigation, TX, WZ, RW, XhangZ, RX, XH, SW, YanhangL, JL, YouxiL, YimanL, ZG, and WL; resources, MC, JL, and YS; data curation, TX, WZ, RW, and LZ; writing-original draft, TX, WZ, RW, and LZ; writing-review and editing, MC, JL, YS, and LZ; supervision, LZ.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Xia, T., Zhang, W., Wu, R. et al. Dermal adipogenesis protects against neutrophilic skin inflammation during psoriasis pathogenesis. Cell Mol Immunol (2025). https://doi.org/10.1038/s41423-025-01296-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41423-025-01296-5