Abstract
Background
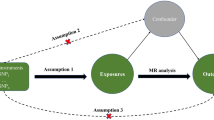
Previous studies have indicated potential associations between metals, lifestyle factors, and sarcopenia. However, the specific causal relationships between iron status, lifestyle factors, and sarcopenia remain unclear. Therefore, we conducted a bidirectional two-sample Mendelian Randomization (MR) approach to investigate these relationships.
Methods
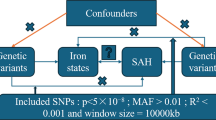
The exposure variables included iron status, living alone, coffee intake, alcohol taken with meals, and moderate physical activity, while the outcome variable was sarcopenia, assessed by grip strength in both hands and usual walking pace. We employed the Weighted Median (WM), the Inverse Variance-Weighted (IVW), and other MR methods to explore these problems for analysis. Simultaneously, we conducted a bidirectional MR analysis to assess whether sarcopenia has a reverse causal relationship with internal iron status.
Results
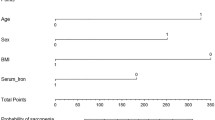
In our present research, we found serum iron (P = 0.033), ferritin (P = 0.001), transferrin saturation (P = 0.029) and coffee intake (P = 0.002) revealed a negative trend for sarcopenia, living alone (P = 0.022) and alcohol taken with meal (P = 0.006) showed a opposite trend for sarcopenia. Whereas sarcopenia showed negative trend for ferritin (P = 0.041) and transferrin saturation (P = 0.043), showed the opposite trend for transferrin (P = 0.021).
Conclusion
Our study suggested that higher serum iron levels might reduce the risk of sarcopenia. Moreover, living alone and alcohol consumption might increase the sarcopenia risk, while coffee intake and moderate physical activity could reduce the sarcopenia risk.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The datasets presented in this study can be found in online repositories. The websites for these datasets have been provided in the article. Further inquiries can be directed to the corresponding author.
References
Cruz-Jentoft AJ, Sayer AA. Sarcopenia. Lancet. 2019;393:2636–46. https://doi.org/10.1016/S0140-6736(19)31138-9.
Petermann-Rocha F, Balntzi V, Gray SR, Lara J, Ho FK, Pell JP. et al. Global prevalence of sarcopenia and severe sarcopenia: a systematic review and meta-analysis. J Cachexia Sarcopenia Muscle. 2022;13:86–99. https://doi.org/10.1002/jcsm.12783.
Gao K, Cao LF, Ma WZ, Gao YJ, Luo MS, Zhu J. et al. Association between sarcopenia and cardiovascular disease among middle-aged and older adults: Findings from the China health and retirement longitudinal study. EClinicalMedicine. 2022;44:101264. https://doi.org/10.1016/j.eclinm.2021.101264.
He N, Zhang Y, Zhang L, Zhang S, Ye H. Relationship between sarcopenia and cardiovascular diseases in the elderly: an overview. Front Cardiovasc Med. 2021;8:743710. https://doi.org/10.3389/fcvm.2021.743710.
Sousa AS, Guerra RS, Fonseca I, Pichel F, Ferreira S, Amaral TF. Financial impact of sarcopenia on hospitalization costs. Eur J Clin Nutr. 2016;70:1046–51. https://doi.org/10.1038/ejcn.2016.73.
Bruyère O, Beaudart C, Ethgen O, Reginster JY, Locquet M. The health economics burden of sarcopenia: a systematic review. Maturitas 2019;119:61–69. https://doi.org/10.1016/j.maturitas.2018.11.003.
Petermann-Rocha F, Ho FK, Welsh P, Mackay D, Brown R, Gill J. et al. Physical capability markers used to define sarcopenia and their association with cardiovascular and respiratory outcomes and all-cause mortality: a prospective study from UK Biobank. Maturitas. 2020;138:69–75. https://doi.org/10.1016/j.maturitas.2020.04.017.
Xie WQ, He M, Yu DJ, Wu YX, Wang XH, Lv S. et al. Mouse models of sarcopenia: classification and evaluation. J Cachexia Sarcopenia Muscle. 2021;12:538–54. https://doi.org/10.1002/jcsm.12709.
Lee SM, Edmonston B. Living Alone Among Older Adults in Canada and the U.S. Healthc (Basel). 2019;7:68. https://doi.org/10.3390/healthcare7020068.
Yang J, Huang J, Yang X, Li S, Wu X, Ma X. The association of living alone and social isolation with sarcopenia: a systematic review and meta-analysis. Ageing Res Rev. 2023;91:102043. https://doi.org/10.1016/j.arr.2023.102043.
Sha T, Li W, He H, Wu J, Wang Y, Li H. Causal relationship of genetically predicted serum micronutrients levels with sarcopenia: a mendelian randomization study. Front Nutr. 2022;9:913155. https://doi.org/10.3389/fnut.2022.913155.
Zhong J, Xie W, Wang X, Dong X, Mo Y, Liu D. et al. The prevalence of sarcopenia among hunan province community-dwelling adults aged 60 years and older and its relationship with lifestyle: diagnostic criteria from the asian working group for sarcopenia 2019 update. Med (Kaunas). 2022;58:1562. https://doi.org/10.3390/medicina58111562.
Muckenthaler MU, Rivella S, Hentze MW, Galy B. A red carpet for iron metabolism. Cell 2017;168:344–61. https://doi.org/10.1016/j.cell.2016.12.034.
McClung JP. Iron, zinc, and physical performance. Biol Trace Elem Res. 2019;188:135–9. https://doi.org/10.1007/s12011-018-1479-7.
Hong SH, Bae YJ. Association between alcohol consumption and the risk of sarcopenia: a systematic review and meta-analysis. Nutrients. 2022;14:3266 https://doi.org/10.3390/nu14163266.
Chen Y, Liu C, Hu M. Association between triglyceride-glucose index and sarcopenia in China: a nationally representative cohort study. Exp Gerontol. 2024;190:112419. https://doi.org/10.1016/j.exger.2024.112419.
Yang J, Liu C, Zhao S, Wang L, Wu G, Zhao Z. et al. The association between the triglyceride-glucose index and sarcopenia: data from the NHANES 2011–2018. Lipids Health Dis. 2024;23:219. https://doi.org/10.1186/s12944-024-02201-1.
Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A. Coffee: biochemistry and potential impact on health. Food Funct. 2014;5:1695–717. https://doi.org/10.1039/c4fo00042k.
Guo Y, Niu K, Okazaki T, Wu H, Yoshikawa T, Ohrui T. et al. Coffee treatment prevents the progression of sarcopenia in aged mice in vivo and in vitro. Exp Gerontol. 2014;50:1–8. https://doi.org/10.1016/j.exger.2013.11.005.
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F. et al. European Working Group on Sarcopenia in Older P. Sarcopenia: european consensus on definition and diagnosis: report of the european working group on sarcopenia in older people. Age Ageing. 2010;39:412–23. https://doi.org/10.1093/ageing/afq034.
Otsuka Y, Yamada Y, Maeda A, Izumo T, Rogi T, Shibata H. et al. Effects of resistance training intensity on muscle quantity/quality in middle-aged and older people: a randomized controlled trial. J Cachexia Sarcopenia Muscle. 2022;13:894–908. https://doi.org/10.1002/jcsm.12941.
Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA. et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: the strobe-mr statement. JAMA. 2021;326:1614–21. https://doi.org/10.1001/jama.2021.18236.
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, BruyŠre O, Cederholm T. et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. https://doi.org/10.1093/ageing/afy169.
Semenova EA, Pranckevičienė E, Bondareva EA, Gabdrakhmanova LJ. Ahmetov II. identification and characterization of genomic predictors of sarcopenia and sarcopenic obesity using uk biobank data. Nutrients. 2023;15:758. https://doi.org/10.3390/nu15030758.
Latunde-Dada GO. Ferroptosis: role of lipid peroxidation, iron and ferritinophagy. Biochim Biophys Acta Gen Subj. 2017;1861:1893–1900. https://doi.org/10.1016/j.bbagen.2017.05.019.
Reardon TF, Allen DG. Iron injections in mice increase skeletal muscle iron content, induce oxidative stress and reduce exercise performance. Exp Physiol. 2009;94:720–30. https://doi.org/10.1113/expphysiol.2008.046045.
Ikeda Y, Imao M, Satoh A, Watanabe H, Hamano H, Horinouchi Y. et al. Iron-induced skeletal muscle atrophy involves an Akt-forkhead box O3-E3 ubiquitin ligase-dependent pathway. J Trace Elem Med Biol. 2016;35:66–76. https://doi.org/10.1016/j.jtemb.2016.01.011.
Vinke JSJ, Gorter AR, Eisenga MF, Dam WA, van der Meer P, van den Born J. et al. Iron deficiency is related to lower muscle mass in community-dwelling individuals and impairs myoblast proliferation. J Cachexia Sarcopenia Muscle. 2023;14:1865–79. https://doi.org/10.1002/jcsm.13277.
Stugiewicz M, Tkaczyszyn M, Kasztura M, Banasiak W, Ponikowski P, Jankowska EA. The influence of iron deficiency on the functioning of skeletal muscles: experimental evidence and clinical implications. Eur J Heart Fail. 2016;18:762–73. https://doi.org/10.1002/ejhf.467.
Mackler B, Grace R, Finch CA. Iron deficiency in the rat: effects on oxidative metabolism in distinct types of skeletal muscle. Pediatr Res. 1984;18:499–500. https://doi.org/10.1203/00006450-198406000-00001.
Henderson SA, Dallman PR, Brooks GA. Glucose turnover and oxidation are increased in the iron-deficient anemic rat. Am J Physiol. 1986;250:E414–E421. https://doi.org/10.1152/ajpendo.1986.250.4.E414.
Thompson CH, Green YS, Ledingham JG, Radda GK, Rajagopalan B. The effect of iron deficiency on skeletal muscle metabolism of the rat. Acta Physiol Scand. 1993;147:85–90. https://doi.org/10.1111/j.1748-1716.1993.tb09475.x.
Baker LA, Cahalin LP, Gerst K, Burr JA. Productive activities and subjective well-being among older adults: the influence of number of activities and time commitment. Soc Indic Res. 2005;73:431–58. https://doi.org/10.1007/s11205-005-0805-6.
Kim DA, Benjamin EJ, Fowler JH, Christakis NA. Social connectedness is associated with fibrinogen level in a human social network. Proc Biol Sci. 2016;283:20160958. https://doi.org/10.1098/rspb.2016.0958.
Koh-Bell A, Chan J, Mann AK, Kapp DS. Social isolation, inflammation and cancer mortality from the national health and nutrition examination survey - a study of 3360 women. BMC Public Health. 2021;21:1289. https://doi.org/10.1186/s12889-021-11352-0.
Dalle S, Rossmeislova L, Koppo K. The role of inflammation in age-related sarcopenia. Front Physiol. 2017;8:1045. https://doi.org/10.3389/fphys.2017.01045.
Avelino J, Cristancho M, Georgiou S, Imbach P, Aguilar L, Bornemann G. et al. The coffee rust crises in Colombia and Central America (2008–2013): impacts, plausible causes and proposed solutions. Food Security. 2015;7:303–21. https://doi.org/10.1007/s12571-015-0446-9.
Costa MS, Botton PH, Mioranzza S, Souza DO, Porciúncula LO. Caffeine prevents age-associated recognition memory decline and changes brain-derived neurotrophic factor and tirosine kinase receptor (TrkB) content in mice. Neuroscience. 2008;153:1071–8. https://doi.org/10.1016/j.neuroscience.2008.03.038.
Horrigan LA, Kelly JP, Connor TJ. Immunomodulatory effects of caffeine: friend or foe?
Hong-Brown LQ, Brown CR, Kazi AA, Huber DS, Pruznak AM, Lang CH. Alcohol and PRAS40 knockdown decrease mTOR activity and protein synthesis via AMPK signaling and changes in mTORC1 interaction. J Cell Biochem. 2010;109:1172–84.
Hong-Brown LQ, Frost RA, Lang CH. Alcohol impairs protein synthesis and degradation in cultured skeletal muscle cells. Alcohol Clin Exp Res. 2001;25:1373–82.
Daily JW, Park S. Sarcopenia is a cause and consequence of metabolic dysregulation in aging humans: effects of gut dysbiosis, glucose dysregulation, diet and lifestyle. Cells. 2022;11:338. https://doi.org/10.3390/cells11030338.
Hetherington MM, Cameron F, Wallis DJ, Pirie LM. Stimulation of appetite by alcohol. Physiol Behav. 2001;74:283–9.
Kovář J, Zemánková K. Moderate alcohol consumption and triglyceridemia.
Li CW, Yu KA-O, Shyh-Chang N, Jiang Z, Liu T, Ma S. et al. Pathogenesis of sarcopenia and the relationship with fat mass: descriptive review. J Cachexia Sarcopenia Muscle. 2022;13:781–94.
Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Stärkel P. et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA. 2014;111:E4485–93.
Zhao JA-O, Huang Y, Yu X. A narrative review of gut-muscle axis and sarcopenia: the potential role of gut microbiota. Int J Gen Med. 2021;14:1263–73.
Dent E, Morley JE, Cruz-Jentoft AJ, Arai H, Kritchevsky SB, Guralnik J. et al. International clinical practice guidelines for sarcopenia (icfsr): screening, diagnosis and management. J Nutr Health Aging. 2018;22:1148–61.
Negm AM, Lee J, Hamidian R, Jones CA, Khadaroo RG. Management of sarcopenia: a network meta-analysis of randomized controlled trials. J Am Med Dir Assoc. 2022;23:707–14.
Acknowledgements
Everyone who contributed significantly to the work has been listed.
Funding
Shandong First Medical University Science and Education Integration talents academic promotion plan funds (922/001003088002), Beijing Municipal Talent Work Leading Group - Young Beijing Scholars Talent Program (Youth Beijing Scholar 2020, No.25), Beijing Hospital Management Center Summit Talent Program (DFL20220401). The article was supported by the visiting research fund for teachers of ordinary undergraduate universities in Shandong Province.
Author information
Authors and Affiliations
Contributions
QZH and YJL conceived the study, QZH and YJL obtained the funding, ZHQ wrote the first draft, QZH and CYH critically revised the manuscript. QZH, CYH and ZHQ made substantial contributions to the design of the protocol. YCZ, XSX, HRZ, JJL, JL and YYZ prepared the figure and table. All authors critically reviewed the draft and approved the final version for publication.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Qiu, Z., Hou, C., Xue, X. et al. The causal relationships between iron status and sarcopenia in Europeans: a bidirectional two-sample Mendelian randomization study. Eur J Clin Nutr 79, 207–213 (2025). https://doi.org/10.1038/s41430-024-01531-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41430-024-01531-8
This article is cited by
-
A Cross-sectional Study on Age-Related Changes in Muscle Iron Deposition and Fat Infiltration: Associations with Grip Strength in a Healthy Adult Cohort
Biological Trace Element Research (2025)