Abstract
Background/objectives
Although polygenic risk scores (PRSs) have been developed for age-related macular degeneration (AMD), it is not known whether these scores are associated with impairment of visual functions in older individuals with healthy macula. We evaluated age-related changes in visual function in people aged 55 years or above with healthy macula and determined the associations of age-related visual function changes with AMD PRS in people with healthy macula.
Subjects/Methods
Participants aged 55 years or above with healthy macula and a comparative group of people with early or intermediate AMD from the Northern Ireland Sensory Ageing study were included. 45 SNPs were included for PRS calculation.
Results
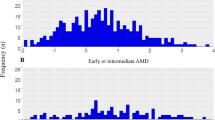
A total of 470 participants with healthy macula were included (Beckman grade 0 or 1). The comparator group consisted of participants with early AMD (n = 87) or intermediate AMD (n = 48). All visual functions except metrics of central visual field assessment showed a significant decline with age in adjusted linear regression models. Rod intercept time (RIT) was the only visual function significantly associated with PRS with Beta = 0.12 (95% confidence interval: 0.01–0.23), P = 0.03. A PRS integrated model achieved the highest area under the receiver operating characteristic curve (AUC) of 0.803 (0.732 to 0.874) to distinguish between normal or increased RIT.
Conclusions and relevance
We observed a significant decline in multiple visual functions with increasing age. However, PRS was significantly associated with RIT only, highlighting the genetic association of age-related decline in rod function.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
269,00 € per year
only 14,94 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
Change history
27 March 2025
The original online version of this article was revised: In this article the article title has been updated to “Polygenic Risk Score Impact Visual Function in Healthy: The Northern Ireland Sensory Ageing Study” because of inoccret grammar/missing words.
03 April 2025
A Correction to this paper has been published: https://doi.org/10.1038/s41433-025-03773-7
References
Klein R, Klein BE, Knudtson MD, Wong TY, Cotch MF, Liu K, et al. Prevalence of age-related macular degeneration in 4 racial/ethnic groups in the multi-ethnic study of atherosclerosis. Ophthalmology. 2006;113:373–80.
Owsley C, McGwin G Jr, Scilley K, Kallies K. Development of a questionnaire to assess vision problems under low luminance in age-related maculopathy. Invest Ophthalmol Vis Sci. 2006;47:528–35.
Mangione CM, Berry S, Spritzer K, Janz NK, Klein R, Owsley C, et al. Identifying the content area for the 51-item National Eye Institute Visual Function Questionnaire: results from focus groups with visually impaired persons. Arch Ophthalmol. 1998;116:227–33.
DeAngelis MM, Owen LA, Morrison MA, Morgan DJ, Li M, Shakoor A, et al. Genetics of age-related macular degeneration (AMD). Hum Mol Genet. 2017;26:R45–r50.
Jabbarpoor Bonyadi MH, Yaseri M, Bonyadi M, Soheilian M, Karimi S. Association of combined complement factor H Y402H and ARMS/LOC387715 A69S polymorphisms with age-related macular degeneration: a meta-analysis. Curr Eye Res. 2016;41:1519–25.
Ulańczyk Z, Grabowicz A, Mozolewska-Piotrowska K, Safranow K, Kawa MP, Pałucha A, et al. Genetic factors associated with age-related macular degeneration: identification of a novel PRPH2 single nucleotide polymorphism associated with increased risk of the disease. Acta Ophthalmol. 2021;99:739–49.
Fritsche LG, Igl W, Bailey JN, Grassmann F, Sengupta S, Bragg-Gresham JL, et al. A large genome-wide association study of age-related macular degeneration highlights contributions of rare and common variants. Nat Genet. 2016;48:134–43.
Colijn JM, Meester-Smoor M, Verzijden T, de Breuk A, Silva R, Merle BMJ, et al. Genetic risk, lifestyle, and age-related macular degeneration in Europe: the EYE-RISK Consortium. Ophthalmology. 2021;128:1039–49.
Lad EM, Fang V, Tessier M, Rautanen A, Gayan J, Stinnett SS, et al. Longitudinal evaluation of visual function impairments in early and intermediate age-related macular degeneration patients. Ophthalmol Sci. 2022;2:100173.
Neville CE, Young IS, Kee F, Hogg RE, Scott A, Burns F, et al. Northern Ireland Cohort for the Longitudinal Study of Ageing (NICOLA): health assessment protocol, participant profile and patterns of participation. BMC Public Health. 2023;23:466.
Higgins BE, Montesano G, Crabb DP, Naskas TT, Graham KW, Chakravarthy U, et al. Assessment of the classification of age-related macular degeneration severity from the Northern Ireland Sensory Ageing Study using a measure of dark adaptation. Ophthalmol Sci. 2022;2:100204.
Hogg RE, Wright DM, Quinn NB, Muldrew KA, Hamill B, Smyth L, et al. Prevalence and risk factors for age-related macular degeneration in a population-based cohort study of older adults in Northern Ireland using multimodal imaging: NICOLA study. Br J Ophthalmol. 2023;107:1873–9.
Rudnicka AR, Jarrar Z, Wormald R, Cook DG, Fletcher A, Owen CG. Age and gender variations in age-related macular degeneration prevalence in populations of European ancestry: a meta-analysis. Ophthalmology. 2012;119:571–80.
Flamendorf J, Agrón E, Wong WT, Thompson D, Wiley HE, Doss EL, et al. Impairments in dark adaptation are associated with age-related macular degeneration severity and reticular pseudodrusen. Ophthalmology. 2015;122:2053–62.
Jackson GR, Edwards JG. A short-duration dark adaptation protocol for assessment of age-related maculopathy. J Ocul Biol Dis Infor. 2008;1:7–11.
DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–45.
Rubin GS, West SK, Muñoz B, Bandeen-Roche K, Zeger S, Schein O, et al. A comprehensive assessment of visual impairment in a population of older Americans. The SEE study. Salisbury Eye Evaluation Project. Invest Ophthalmol Vis Sci. 1997;38:557–68.
Mullins RF, McGwin G Jr., Searcey K, Clark ME, Kennedy EL, Curcio CA, et al. The ARMS2 A69S polymorphism is associated with delayed rod-mediated dark adaptation in eyes at risk for incident age-related macular degeneration. Ophthalmology. 2019;126:591–600.
Curcio CA, Medeiros NE, Millican CL. Photoreceptor loss in age-related macular degeneration. Invest Ophthalmol Vis Sci. 1996;37:1236–49.
Goodman C, Podolsky RH, Childers KL, Roberts R, Katz R, Waseem R, et al. Do multiple physiological OCT biomarkers indicate age-related decline in rod mitochondrial function in C57BL/6J mice? Front Neurosci. 2023;17:1280453.
Kaye RA, Patasova K, Patel PJ, Hysi P, Lotery AJ. Macular thickness varies with age-related macular degeneration genetic risk variants in the UK Biobank cohort. Sci Rep. 2021;11:23255.
Kamoshita M, Ozawa Y, Kubota S, Miyake S, Tsuda C, Nagai N, et al. AMPK-NF-κB axis in the photoreceptor disorder during retinal inflammation. PLoS ONE. 2014;9:e103013.
Ferris FL 3rd, Wilkinson CP, Bird A, Chakravarthy U, Chew E, et al. Clinical classification of age-related macular degeneration. Ophthalmology. 2013;120:844–51.
Grewal MK, Chandra S, Gurudas S, Rasheed R, Sen P, Menon D, et al. Functional clinical endpoints and their correlations in eyes with AMD with and without subretinal drusenoid deposits-a pilot study. Eye. 2022;36:398–406.
Domalpally A, Agrón E, Pak JW, Keenan TD, Ferris FL 3rd, Clemons TE, et al. Prevalence, risk, and genetic association of reticular pseudodrusen in age-related macular degeneration: Age-Related Eye Disease Study 2 Report 21. Ophthalmology. 2019;126:1659–66.
Zekavat SM, Sekimitsu S, Ye Y, Raghu V, Zhao H, Elze T, et al. Photoreceptor layer thinning is an early biomarker for age-related macular degeneration: epidemiologic and genetic evidence from UK Biobank OCT data. Ophthalmology. 2022;129:694–707.
Curcio CA, Kar D, Owsley C, Sloan KR, Ach T. Age-related macular degeneration, a mathematically tractable disease. Invest Ophthalmol Vis Sci. 2024;65:4.
Curcio CA, Millican CL, Bailey T, Kruth HS. Accumulation of cholesterol with age in human Bruch’s membrane. Invest Ophthalmol Vis Sci. 2001;42:265–74.
Smith RT, Olsen TW, Chong V, Kim J, Hammer M, Lema G. et al. Subretinal drusenoid deposits, age-related macular degeneration, and cardiovascular disease. Asia Pac J Ophthalmol. 2024;13:100036.
Owsley C, Swain TA, McGwin G Jr., Clark ME, Kar D, Crosson JN, et al. How vision is impaired from aging to early and intermediate age-related macular degeneration: insights from ALSTAR2 baseline. Transl Vis Sci Technol. 2022;11:17.
Lin LY, Zhou Q, Hagstrom S, Maguire MG, Daniel E, Grunwald JE, et al. Association of single-nucleotide polymorphisms in age-related macular degeneration with pseudodrusen: secondary analysis of data from the comparison of AMD treatments trials. JAMA Ophthalmol. 2018;136:682–8.
Funding
Atlantic Philanthropies, the Economic and Social Research Council, the UKCRC Centre of Excellence for Public Health Northern Ireland, the Centre for Ageing Research and Development in Ireland, the Office of the First Minister and Deputy First Minister, the Health and Social Care Research and Development Division of the Public Health Agency, the Wellcome Trust/Wolfson Foundation, Fight for Sight (Ref No.: 1906), and Queen’s University Belfast provide core financial support for the Northern Ireland Cohort of Longitudinal Study of Ageing study. The authors alone are responsible for the interpretation of the data, and any views or opinions presented are solely those of the authors and do not necessarily represent those of the Northern Ireland Cohort of Longitudinal Study of Ageing study team. The Northern Ireland Sensory Ageing study was funded by grants from the College of Optometrists, Diabetes UK, Macular Society, RNIB, Guide Dogs for the Blind, Thomas Pocklington Trust and the Belfast Association for the Blind. Role of the Sponsor: The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
SS, REH: research design. FYT: data analysis, interpretation, REH, BEH, DMW, LS: research execution. All authors contributed towards the preparation of the manuscript and approved the final submitted version. The corresponding author is solely responsible for managing communication between co-authors; that all authors are included in the author list; order has been agreed by all authors; and that all authors are aware that the paper was submitted.
Corresponding author
Ethics declarations
Competing interests
SS is a member of the Eye editorial board.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: In this article the article title has been updated to “Polygenic Risk Score Impact Visual Function in Healthy: The Northern Ireland Sensory Ageing Study” because of inoccret grammar/missing words.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tang, F., Hogg, R.E., Higgins, B.E. et al. Polygenic Risk Score Impact on Visual Function in Older Individuals with Healthy Macula: The Northern Ireland Sensory Ageing Study. Eye 39, 1508–1516 (2025). https://doi.org/10.1038/s41433-025-03642-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-025-03642-3