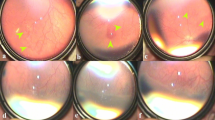
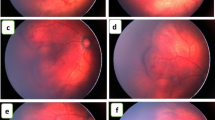
Abstract
Purpose
To compare the safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in treatment of Retinopathy of prematurity (ROP)
Methods
Retrospective study that included newborns with type 1 ROP who received intravitreal Bevacizumab (Avastin) or Ranibizumab (Accentrix) or Ranibizumab biosimilar (Razumab). Babies were followed up as per protocol and retreatment with laser or repeat anti vascular endothelial growth factor (VEGF) was done in case of reactivation and surgery in case of progression to traction. Outcome measures include need for retreatment, proportion of eyes achieving vascularization up to ora and adverse events
Results
148 eyes of 75 babies, received intravitreal injection of which 139 eyes of 70 babies were included in our analysis. 68 eyes received bevacizumab (IVB), 31 eyes received Accentrix (IVA), 40 eyes received Razumab(IVR). The rate of retreatment was 17.6%, 32.2% and 25% for IVB, IVA and IVR respectively (p = 0.1). IVB group showed a significantly delayed reactivation (p < 0.001), while Ranibizumab and its biosimilar Razumab were comparable (p = 0.17). Vascularization up to ora was observed in 60%, 61% and 50% eyes (p = 0.76) at a median of 27, 28 and 24 weeks post-treatment (p = 0.09) in IVB, IVA and IVR groups respectively. No eyes developed intraocular inflammation or cataract.
Conclusion
All three drugs were similar in their efficacy and safety profile with bevacizumab showing a later reactivation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 18 print issues and online access
269,00 € per year
only 14,94 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
Data availability
The data pertaining to this manuscript are included in the article and any additional data can be made available on request.
References
Early Treatment For Retinopathy Of Prematurity Cooperative Group. Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121:1684–94.
Shah PK, Narendran V, Tawansy KA, Raghuram A, Narendran K. Intravitreal bevacizumab (Avastin) for post laser anterior segment ischemia in aggressive posterior retinopathy of prematurity. Indian J Ophthalmol. 2007;55:75–6.
Chiang MF, Quinn GE, Fielder AR, Ostmo SR, Paul Chan RV, Berrocal A, et al. International classification of retinopathy of prematurity, Third Edition. Ophthalmology. 2021;128:e51–e68.
Cryotherapy for Retinopathy of Prematurity Cooperative Group. Multicenter trial of cryotherapy for retinopathy of prematurity. Preliminary results. Arch Ophthalmol. 1988;106:471–9.
Pierce EA, Foley ED, Smith LE. Regulation of vascular endothelial growth factor by oxygen in a model of retinopathy of prematurity. Arch Ophthalmol. 1996;114:1219–28.
Kong L, Bhatt AR, Demny AB, Coats DK, Li A, Rahman EZ, et al. Pharmacokinetics of bevacizumab and its effects on serum VEGF and IGF-1 in infants with retinopathy of prematurity. Invest Ophthalmol Vis Sci. 2015;56:956–61.
Fidler M, Fleck BW, Stahl A, Marlow N, Chastain JE, Li J, et al. RAINBOW study group†. Ranibizumab population pharmacokinetics and free VEGF pharmacodynamics in preterm infants with retinopathy of prematurity in the RAINBOW trial. Transl Vis Sci Technol. 2020;9:43.
Enríquez AB, Avery RL, Baumal CR. Update on anti-vascular endothelial growth factor safety for retinopathy of prematurity. Asia Pac J Ophthalmol. 2020;9:358–68.
Patel NA, Acaba-Berrocal LA, Hoyek S, Fan KC, Martinez-Castellanos MA, Baumal CR, et al. Retinopathy of prematurity injection consortium. practice patterns and outcomes of intravitreal anti-VEGF injection for retinopathy of prematurity: an international multicenter study. Ophthalmology. 2022;129:1380–8.
Xu L, Lu T, Tuomi L, Jumbe N, Lu J, Eppler S, et al. Pharmacokinetics of ranibizumab in patients with neovascular age-related macular degeneration: a population approach. Invest Ophthalmol Vis Sci. 2013;54:1616–24.
Avery RL, Castellarin AA, Steinle NC, Dhoot DS, Pieramici DJ, See R, et al. Systemic Pharmacokinetics and Pharmacodynamics of Intravitreal Aflibercept, Bevacizumab, and Ranibizumab. Retina. 2017;37:1847–58.
Wu WC, Shih CP, Lien R, Wang NK, Chen YP, Chao AN, et al. Serum vascular endothelial growth factor after bevacizumab or ranibizumab treatment for retinopathy of prematurity. Retina. 2017;37:694–701.
Stahl A, Lepore D, Fielder A, Fleck B, Reynolds JD, Chiang MF, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): an open-label randomised controlled trial. Lancet. 2019;394:1551–9.
Darwish D, Chee RI, Patel SN, Jonas K, Ostmo S, Campbell JP, et al. Anti-Vascular Endothelial Growth Factor and the Evolving Management Paradigm for Retinopathy of Prematurity. Asia Pac. J Ophthalmol. 2018;7:136–44.
Celiker H, Sahin O. Angiographic findings in cases with a history of severe retinopathy of prematurity treated with anti-VEGFs: Follow-up to age 6 years. Int Ophthalmol. 2022;42:1317–37.
Chan JJT, Lam CPS, Kwok MKM, Wong RLM, Lee GKY, Lau WWY, et al. Risk of recurrence of retinopathy of prematurity after initial intravitreal ranibizumab therapy. Sci Rep. 2016;6:27082.
Adams GG, Bunce C, Xing W, Butler L, Long V, Reddy A, et al. Retinopathy of prematurity in the United Kingdom:Retreatment rates, visual and structural 1-year outcomes. Eye. 2018;32:1752–9.
Ji MH, Moshfeghi DM, Shields RA, Bodnar Z, Ludwig CA, Callaway NF, et al. Conserved regression patterns of retinopathy of prematurity after intravitreal ranibizumab:A class effect. Eur J Ophthalmol. 2021;31:2135–40.
Lyu J, Zhang Q, Chen CL, Xu Y, Ji XD, Li JK, et al. Recurrence of retinopathy of prematurity after intravitreal ranibizumab monotherapy:Timing and risk factors. Invest Ophthalmol Vis Sci. 2017;58:1719–25.
Blair M, Gonzalez JMG, Snyder L, Schechet S, Greenwald M, Shapiro M, et al. Bevacizumab or laser for aggressive posterior retinopathy of prematurity. Taiwan J Ophthalmol. 2018;8:243–8.
Tong Q, Yin H, Zhao M, Li X, Yu W. Outcomes and prognostic factors for aggressive posterior retinopathy of prematurity following initial treatment with intravitreal ranibizumab. BMC Ophthalmol. 2018;18:150.
Blair M, Rodriguez S, Schechet S, ShapiroW M. Aggressive Posterior Retinopathy of Prematurity (APROP). in C Wu, W-C Lam (eds.), A Quick Guide to Pediatric Retina (First edition, 43-51) Springer Nature Singapore Pte Ltd. 2021
Mintz-Hittner HA, Kennedy KA, Chuang AZ. BEAT-ROP Cooperative Group. Efficacy of intravitreal bevacizumab for stage 3+ retinopathy of prematurity. N Engl J Med. 2011;364:603–15.
Ekinci DY, Vural AD, Bayramoglu SE, Onur IU, Hergunsel GO. Assessment of vascular leakage and its development with FFA among patients treated with intravitreal anti-VEGF due to aggressive posterior ROP. Int Ophthalmol. 2019;39:2697–705.
Diggikar S, Gurumoorthy P, Trif P, Mudura D, Nagesh NK, Galis R, et al. Retinopathy of prematurity and neurodevelopmental outcomes in preterm infants: A systematic review and meta-analysis. Front Pediatr. 2023;11:1055813.
Marlow N, Reynolds JD, Lepore D, Fielder AR, Stahl A, Hao H, et al. Ranibizumab versus laser therapy for the treatment of very low birthweight infants with retinopathy of prematurity (RAINBOW): five-year outcomes of a randomised trial. EClinicalMedicine. 2024;71:102567.
Honda S, Hirabayashi H, Tsukahara Y, Negi A. Acute contraction of the proliferative membrane after an intravitreal injection of bevacizumab for advanced retinopathy of prematurity. Graefes Arch Clin Exp Ophthalmol. 2008;246:1061–3.
Zepeda-Romero L, Liera-Garcia J, Gutiérrez-Padilla J, et al. Paradoxical vascular–fibrotic reaction after intravitreal bevacizumab for retinopathy of prematurity. Eye. 2010;24:931–3.
Yonekawa Y, Wu WC, Nitulescu CE, Chan RVP, Thanos A, Thomas BJ, et al. Progressive retinal detachment in infants with retinopathy of prematurity treated with intravitreal bevacizumab or ranibizumab. Retina. 2018;38:1079–83.
Maitra P, Prema S, Narendran V, Shah PK. Safety and efficacy of intravitreal anti vascular endothelial growth factor for severe posterior retinopathy of prematurity with flat fibrovascular proliferation. World J Clin Pediatr. 2023;12:220–9.
Wood EH, Rao P, Moysidis SN, Dedania VS, Elman MJ, Drenser KA, et al. Fellow Eye Anti-VEGF ‘Crunch’ Effect in Retinopathy of Prematurity. Ophthalmic Surg Lasers Imaging Retina. 2018;49:e102–e104.
Guidelines on evaluation of biosimilars. http://www.who.int/publications/m/item/guidelines-on-evaluation-of-biosimilars; Accessed June 10, 2024
Prajapati V, Choudhary T, Chauhan W, Shah S, Handa R, Jahan B, et al. Efficacy of a biosimilar ranibizumab monotherapy for the treatment of retinopathy of prematurity. Indian J Ophthalmol. 2023;71:411–5.
Ratra D, Roy K, Giridhar S, Madaan S, Sankara Nethralaya Vitreoretinal Study Group. Comparison between ranibizumab biosimilar, innovator ranibizumab and bevacizumab in a real-world situation. Ophthalmol Ther. 2022;11:135–149.
Sharma A, Hafeez Faridi M, Kumar N, Parachuri N, Sharma R, Kuppermann BD, et al. Immunogenicity and efficacy after switching from original Ranibizumab to a Ranibizumab biosimilar: real-world data. Eye. 2020;34:1008–9.
Chakraborty S, Sheth JU. Efficacy of an Indian Bevacizumab BIOSimilar (BEVATAS) for Type 1 and Aggressive Posterior Retinopathy of Prematurity (BIOS-ROP Study). Clin Ophthalmol. 2024;18:61–68.
Shukla R, Murthy GVS, Gilbert C, Vidyadhar B, Mukpalkar S. Operational guidelines for ROP in India: A summary. Indian J Ophthalmol. 2020;68:S108–S114.
Author information
Authors and Affiliations
Contributions
All Authors have made direct and substantial contributions to the manuscript. Concept and design: Parag K. Shah, Data collection: Sujay Jaju, Kushal U. Agrawal, Abhishek Das, Prema Subramaniam, Analysis an interpretation: Puja Maitra, Manuscript writing: Puja Maitra Manuscript editing/ revision: Parag K. Shah, Supervision: Parag K. Shah, Narendran Venkatapathy.
Corresponding author
Ethics declarations
Competing interests
The authors declares that there is no conflict of interest regarding the publication of this article.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Maitra, P., Jaju, S., Agrawal, K.U. et al. Comparing safety and efficacy of Bevacizumab, Ranibizumab and Ranibizumab biosimilar in Retinopathy of prematurity. Eye 39, 1688–1693 (2025). https://doi.org/10.1038/s41433-025-03735-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41433-025-03735-z