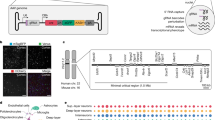
Abstract
Heterozygous mutations in GNAO1 cause an ultra-rare neurodevelopmental disease called GNAO1 encephalopathy, characterized by infantile epilepsy and movement disorder. Here, we provide a functional characterization of the hotspot mutation GNAO1 c.607G>A (p.G203R) and conduct early-phase development of an adeno-associated virus (AAV)-mediated gene therapy approach. The GNAO1 gene encodes the Gαo protein that is involved in neuronal signaling. We showed that the Gαo-G203R lost its ability to enhance forskolin-stimulated cAMP synthesis in HEK293T cells. In primary neuronal culture, Gαo-G203R had a dominant-negative effect on neuronal activity and GABAB-dependent synaptic release. To ablate the mutant protein, we used selective silencing of the pathogenic variant using effectors of RNA interference (RNAi). We selected the short hairpin RNA (sh1500) that suppressed the c.607G>A transcripts, resulting in a 3.8-fold increase in the ratio of wild-type to mutant GNAO1 transcripts in patient-specific neurons. We also detected off-target effects of sh1500 as well as transcriptome changes associated with AAV transduction and RNAi activation. We improved the AAV construct by using an artificial miRNA (miR1500) and the neuron-specific hSyn promoter. Systemic administration of AAV9-hSyn-miR1500 did not cause pathological changes in Gnao1-GGA mice with a “humanized” target sequence. Importantly, AAV9 transduced Gαo-positive neurons in the striatum, thalamus, substantia nigra, and cerebellum, which we defined as primary targets for gene therapy. Our findings pave the road toward the development of AAV-RNAi approaches for dominant-negative GNAO1 variants.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 6 print issues and online access
269,00 € per year
only 44,83 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
The data generated during this study are available within the published article and its supplementary files, or from the corresponding author on reasonable request. The gene expression dataset is available on the NCBI GEO repository with the accession number GSE263736.
References
Nakamura K, Kodera H, Akita T, Shiina M, Kato M, Hoshino H, et al. De Novo mutations in GNAO1, encoding a Gαo subunit of heterotrimeric G proteins, cause epileptic encephalopathy. Am J Hum Genet. 2013;93:496–505.
EuroEPINOMICS-RES Consortium, Epilepsy Phenome/Genome Project, Epi4K Consortium. De novo mutations in synaptic transmission genes including DNM1 cause epileptic encephalopathies. Am J Hum Genet. 2014;95:360–70.
GNAO1[gene] - ClinVar - NCBI. 2024. https://www.ncbi.nlm.nih.gov/clinvar/?term=GNAO1%5Bgene%5D.
Novelli M, Galosi S, Zorzi G, Martinelli S, Capuano A, Nardecchia F, et al. GNAO1-related movement disorder: an update on phenomenology, clinical course, and response to treatments. Park Relat Disord. 2023;111:105405.
JoJo Yang Q-Z, Porter BE, Axeen ET. GNAO1-related neurodevelopmental disorder: literature review and caregiver survey. Epilepsy Behav Rep. 2023;21:100582.
Polikarpova AV, Egorova TV, Lunev EA, Tsitrina AA, Vassilieva SG, Savchenko IM, et al. CRISPR/Cas9-generated mouse model with humanizing single-base substitution in the Gnao1 for safety studies of RNA therapeutics. Front Genome Ed. 2023;5.Frontiers2024. https://www.frontiersin.org/articles/10.3389/fgeed.2023.1034720.
Wettschureck N, Offermanns S. Mammalian G proteins and their cell type specific functions. Physiol Rev. 2005;85:1159–204.
Logothetis DE, Kurachi Y, Galper J, Neer EJ, Clapham DE. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987;325:321–6.
Zamponi GW, Currie KPM. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim Biophys Acta. 2013;1828:1629–43.
Muntean BS, Masuho I, Dao M, Sutton LP, Zucca S, Iwamoto H, et al. Gαo is a major determinant of cAMP signaling in the pathophysiology of movement disorders. Cell Rep. 2021;34:108718.
Solis GP, Bilousov O, Koval A, Lüchtenborg A-M, Lin C, Katanaev VL. Golgi-resident Gαo promotes protrusive membrane dynamics. Cell. 2017;170:939–55.e24.
Feng H, Yuan Y, Williams MR, Roy AJ, Leipprandt JR, Neubig RR. Mice with monoallelic GNAO1 loss exhibit reduced inhibitory synaptic input to cerebellar Purkinje cells. J Neurophysiol. 2022;127:607–22.
Larasati YA, Savitsky M, Koval A, Solis GP, Valnohova J, Katanaev VL. Restoration of the GTPase activity and cellular interactions of Gαo mutants by Zn2+ in GNAO1 encephalopathy models. Sci Adv. 2022;8:eabn9350.
Knight KM, Obarow EG, Wei W, Mani S, Esteller MI, Cui M, et al. Molecular annotation of G protein variants in a neurological disorder. Cell Rep. 2023;42. www.cell.com2024. https://www.cell.com/cell-reports/abstract/S2211-1247(23)01474-2.
Feng H, Sjögren B, Karaj B, Shaw V, Gezer A, Neubig RR. Movement disorder in GNAO1 encephalopathy associated with gain-of-function mutations. Neurology. 2017;89:762–70.
Wang D, Dao M, Muntean BS, Giles AC, Martemyanov KA, Grill B. Genetic modeling of GNAO1 disorder delineates mechanisms of Gαo dysfunction. Hum Mol Genet. 2021;31:510–22.
Solis GP, Koval A, Valnohova J, Kazemzadeh A, Savitsky M, Katanaev VL Neomorphic Gαo mutations gain interaction with Ric8 proteins in GNAO1 encephalopathies. J Clin Invest. 2024;134. www.jci.org2024. https://www.jci.org/articles/view/172057.
Taira R, Akamine S, Okuzono S, Fujii F, Hatai E, Yonemoto K, et al. Gnao1 is a molecular switch that regulates the Rho signaling pathway in differentiating neurons. Sci Rep. 2024;14:17097.
Martier R, Konstantinova P. Gene Therapy for neurodegenerative diseases: slowing down the ticking clock. Front Neurosci. 2020;14. https://www.frontiersin.org/articles/10.3389/fnins.2020.580179.
Ros XB-D, Gu S. Guidelines for the optimal design of miRNA-based shRNAs. Methods San Diego Calif. 2016;103:157–66.
GPP Web Portal—Design hairpins. 2024. https://portals.broadinstitute.org/gpp/public/seq/search.
Klementieva NV, Lunev EA, Shmidt AA, Loseva EM, Savchenko IM, Svetlova EA, et al. RNA interference effectors selectively silence the pathogenic variant GNAO1 c.607 G>A in vitro. Nucleic Acid Ther. 2024. liebertpub.com (Atypon) 2024. https://www.liebertpub.com/doi/abs/10.1089/nat.2023.0043.
Fellmann C, Hoffmann T, Sridhar V, Hopfgartner B, Muhar M, Roth M, et al. An optimized microRNA backbone for effective single-copy RNAi. Cell Rep. 2013;5:1704–13.
Lunev EA, Shmidt AA, Vassilieva SG, Savchenko IM, Loginov VA, Marina VI, et al. Effective viral delivery of genetic constructs to neuronal culture for modeling and gene therapy of GNAO1 encephalopathy. Mol Biol. 2022;56:559–71.
Sokolov RA, Mukhina IV. Spontaneous Ca2+ events are linked to the development of neuronal firing during maturation in mice primary hippocampal culture cells. Arch Biochem Biophys. 2022;727:109330.
Benedetti MC, D’andrea T, Colantoni A, Silachev D, de Turris V, Boussadia Z, et al. Cortical neurons obtained from patient-derived iPSCs with GNAO1 p.G203R variant show altered differentiation and functional properties. Heliyon. 2024;10:e26656.
Vigont VA, Grekhnev DA, Lebedeva OS, Gusev KO, Volovikov EA, Skopin AY, et al. STIM2 mediates excessive store-operated calcium entry in patient-specific iPSC-derived neurons modeling a Juvenile form of Huntington’s disease. Front Cell Dev Biol. 2021;9:625231.
Starikova AV, Skopenkova VV, Polikarpova AV, Reshetov DA, Vassilieva SG, Velyaev OA, et al. Therapeutic potential of highly functional codon-optimized microutrophin for muscle-specific expression. Sci Rep. 2022;12:848.
Ellis BL, Hirsch ML, Barker JC, Connelly JP, Steininger RJ, Porteus MH. A survey of ex vivo/in vitro transduction efficiency of mammalian primary cells and cell lines with Nine natural adeno-associated virus (AAV1-9) and one engineered adeno-associated virus serotype. Virol J. 2013;10:74.
Martier R, Liefhebber JM, García-Osta A, Miniarikova J, Cuadrado-Tejedor M, Espelosin M, et al. Targeting RNA-mediated toxicity in C9orf72 ALS and/or FTD by RNAi-based gene therapy. Mol Ther - Nucleic Acids. 2019;16:26–37.
Patel TP, Man K, Firestein BL, Meaney DF. Automated quantification of neuronal networks and single-cell calcium dynamics using calcium imaging. J Neurosci Methods. 2015;243:26–38.
Berry ASF, Farias Amorim C, Berry CL, Syrett CM, English ED, Beiting DP. An Open-source toolkit to expand bioinformatics training in infectious diseases. mBio. 2021;12. https://doi.org/10.1128/mbio.01214-21.
Alkan F, Wenzel A, Palasca O, Kerpedjiev P, Rudebeck AF, Stadler PF, et al. RIsearch2: suffix array-based large-scale prediction of RNA–RNA interactions and siRNA off-targets. Nucleic Acids Res. 2017;45:e60.
Aurnhammer C, Haase M, Muether N, Hausl M, Rauschhuber C, Huber I, et al. Universal real-time PCR for the detection and quantification of adeno-associated virus serotype 2-derived inverted terminal repeat sequences. Hum Gene Ther Methods. 2012;23:18–28.
Yang L, Wang S, Tang L, Liu B, Ye W, Wang L, et al. Universal stem-loop primer method for screening and quantification of MicroRNA. PLOS ONE. 2014;9:e115293.
Allen Reference Atlases: Atlas Viewer. 2024. http://atlas.brain-map.org/.
Brust TF, Conley JM, Watts VJ. Gαi/o-coupled receptor-mediated sensitization of adenylyl cyclase: 40 years later. Eur J Pharmacol. 2015;763:223–32.
Harrison NL. On the presynaptic action of baclofen at inhibitory synapses between cultured rat hippocampal neurones. physoc.onlinelibrary.wiley.com 2024. https://physoc.onlinelibrary.wiley.com. https://doi.org/10.1113/jphysiol.1990.sp017993.
Gu S, Zhang Y, Jin L, Huang Y, Zhang F, Bassik MC, et al. Weak base pairing in both seed and 3’ regions reduces RNAi off-targets and enhances si/shRNA designs. Nucleic Acids Res. 2014;42:12169–76.
Meneghello G, Verheyen A, Van Ingen M, Kuijlaars J, Tuefferd M, Van Den Wyngaert I, et al. Evaluation of established human iPSC-derived neurons to model neurodegenerative diseases. Neuroscience. 2015;301:204–12.
Duong TT, Lim J, Vasireddy V, Papp T, Nguyen H, Leo L, et al. Comparative AAV-eGFP transgene expression using vector serotypes 1–9, 7m8, and 8b in human pluripotent stem cells, RPEs, and human and rat cortical neurons. Stem Cells Int. 2019;2019:7281912.
Ling Q, Herstine JA, Bradbury A, Gray SJ. AAV-based in vivo gene therapy for neurological disorders. Nat Rev Drug Discov. 2023;22:789–806.
Banning A, Zakrzewicz A, Chen X, Gray SJ, Tikkanen R. Knockout of the CMP-sialic acid transporter SLC35A1 in human cell lines increases transduction efficiency of adeno-associated virus 9: implications for gene therapy potency assays. Cells. 2021;10:1259.
Ansari AM, Ahmed AK, Matsangos AE, Lay F, Born LJ, Marti G, et al. Cellular GFP toxicity and immunogenicity: potential confounders in in vivo cell tracking experiments. Stem Cell Rev. 2016;12:553.
Haldrup SH, Fabian-Jessing BK, Jakobsen TS, Lindholm AB, Adsersen RL, Aagaard L, et al. Subretinal AAV delivery of RNAi-therapeutics targeting VEGFA reduces choroidal neovascularization in a large animal model. Mol Ther Methods Clin Dev. 2024;32. www.cell.com2024. https://www.cell.com/molecular-therapy-family/methods/abstract/S2329-0501(24)00058-5.
Johnston S, Parylak SL, Kim S, Mac N, Lim C, Gallina I, et al. AAV ablates neurogenesis in the adult murine hippocampus. eLife. 2021;10:e59291.
Penaud-Budloo M, François A, Clément N, Ayuso E. Pharmacology of recombinant adeno-associated virus production. Mol Ther Methods Clin Dev. 2018;8:166–80.
Earley LF, Conatser LM, Lue VM, Dobbins AL, Li C, Hirsch ML, et al. Adeno-associated virus serotype-specific inverted terminal repeat sequence role in vector transgene expression. Hum Gene Ther. 2020;31:151.
Keiser MS, Ranum PT, Yrigollen CM, Carrell EM, Smith GR, Muehlmatt AL, et al. Toxicity after AAV delivery of RNAi expression constructs into nonhuman primate brain. Nat Med. 2021;27:1982–9.
Günther A, Luczak V, Abel T, Baumann A. Caspase-3 and GFAP as early markers for apoptosis and astrogliosis in shRNA-induced hippocampal cytotoxicity. J Exp Biol. 2017;220:1400–4.
Szalai R, Till A, Szabo A, Melegh B, Hadzsiev K, Czako M. Overlapping interstitial deletions of the region 9q22.33 to 9q33.3 of three patients allow pinpointing candidate genes for epilepsy and cleft lip and palate. Mol Syndromol. 2023;14:109–22.
Chen S, Li Y, Zhu Y, Fei J, Song L, Sun G, et al. SERPINE1 overexpression promotes malignant progression and poor prognosis of gastric cancer. J Oncol. 2022;2022:2647825.
Cha HL, Choi J-M, Oh H-H, Bashyal N, Kim S-S, Birnbaumer L, et al. Deletion of the α subunit of the heterotrimeric Go protein impairs cerebellar cortical development in mice. Mol Brain. 2019;12.PubMed Central2020. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6585000/.
Roldán-Sastre A, Aguado C, Martín-Belmonte A, Alfaro-Ruiz R, Moreno-Martínez AE, Luján R. Cellular diversity and differential subcellular localization of the G-protein Gαo subunit in the mouse cerebellum. Front Neuroanat. 2021;15.Frontiers2024. https://www.frontiersin.org/journals/neuroanatomy/articles/10.3389/fnana.2021.686279/full.
Hudry E, Vandenberghe LH. Therapeutic AAV gene transfer to the nervous system: a clinical reality. Neuron. 2019;101:839–62.
Pupo A, Fernández A, Low SH, François A, Suárez-Amarán L, Samulski RJ. AAV vectors: the Rubik’s cube of human gene therapy. Mol Ther J Am Soc. Gene Ther. 2022;30:3515–41.
McBride JL, Boudreau RL, Harper SQ, Staber PD, Monteys AM, Martins I, et al. Artificial miRNAs mitigate shRNA-mediated toxicity in the brain: Implications for the therapeutic development of RNAi. Proc Natl Acad Sci USA. 2008;105:5868–73.
Martin JN, Wolken N, Brown T, Dauer WT, Ehrlich ME, Gonzalez-Alegre P. Lethal toxicity caused by expression of shRNA in the mouse striatum: implications for therapeutic design. Gene Ther. 2011;18:666–73.
Kotowska-Zimmer A, Pewinska M, Olejniczak M. Artificial miRNAs as therapeutic tools: challenges and opportunities. Wiley Interdiscip Rev RNA. 2021;12:e1640.
Hollidge BS, Carroll HB, Qian R, Fuller ML, Giles AR, Mercer AC, et al. Kinetics and durability of transgene expression after intrastriatal injection of AAV9 vectors. Front Neurol. 2022;13:1051559.
Champy M, Selloum M, Piard L, Zeitler V, Caradec C, Chambon P, et al. Mouse functional genomics requires standardization of mouse handling and housing conditions. Mamm Genome. 2004;15:768–83.
Silachev D, Koval A, Savitsky M, Padmasola G, Quairiaux C, Thorel F, et al. Mouse models characterize GNAO1 encephalopathy as a neurodevelopmental disorder leading to motor anomalies: from a severe G203R to a milder C215Y mutation. Acta Neuropathol Commun. 2022;10:9.
Akamine S, Okuzono S, Yamamoto H, Setoyama D, Sagata N, Ohgidani M, et al. GNAO1 organizes the cytoskeletal remodeling and firing of developing neurons. FASEB J. 2020;34:16601–21.
Lunev E, Karan A, Egorova T, Bardina M. Adeno-associated viruses for modeling neurological diseases in animals: achievements and prospects. Biomedicines. 2022;10:1140.
Xu J, Peng Q, Cai J, Shangguan J, Su W, Chen G, et al. The Schwann cell-specific G-protein Gαo (Gnao1) is a cell-intrinsic controller contributing to the regulation of myelination in peripheral nerve system. Acta Neuropathol Commun. 2024;12:24.
Liu Z, Zhang J, Wu L, Liu J, Zhang M. Overexpression of GNAO1 correlates with poor prognosis in patients with gastric cancer and plays a role in gastric cancer cell proliferation and apoptosis. Int J Mol Med. 2014;33:589–96.
Inazumi H, Kuwahara K, Nakagawa Y, Kuwabara Y, Numaga-Tomita T, Kashihara T, et al. NRSF-GNAO1 pathway contributes to the regulation of cardiac Ca2+ homeostasis. Circ Res. 2022;130:234–48.
Galosi S, Novelli M, Di Rocco M, Flex E, Messina E, Pollini L, et al. GNAO1 haploinsufficiency: the milder end of the GNAO1 phenotypic spectrum. Mov Disord. 2023;38:2313–4.
UniQure Biopharma BV. A Phase I/II, Randomized, Double-Blind, Sham Control Study to Explore Safety, Tolerability, and Efficacy Signals of Multiple Doses of Striatally-Administered rAAV5-miHTT Total Huntingtin Gene (HTT) Lowering Therapy (AMT-130) in Early Manifest Huntington’s Disease. clinicaltrials.gov, 2023clinicaltrials.gov2024. https://clinicaltrials.gov/study/NCT04120493.
Amado DA, Davidson BL. Gene therapy for ALS: a review. Mol Ther. 2021;29:3345–58.
Martier R, Sogorb-Gonzalez M, Stricker-Shaver J, Hübener-Schmid J, Keskin S, Klima J, et al. Development of an AAV-based MicroRNA gene therapy to treat Machado-Joseph disease. Mol Ther Methods Clin Dev. 2019;15:343–58.
Parrini M, Naskar S, Alberti M, Colombi I, Morelli G, Rocchi A, et al. Restoring neuronal chloride homeostasis with anti-NKCC1 gene therapy rescues cognitive deficits in a mouse model of Down syndrome. Mol Ther. 2021;29:3072–92.
Aimiuwu OV, Fowler AM, Sah M, Teoh JJ, Kanber A, Pyne NK, et al. RNAi-based gene therapy rescues developmental and epileptic encephalopathy in a genetic mouse model. Mol Ther. 2020;28:1706–16.
Miniarikova J, Zanella I, Huseinovic A, van der Zon T, Hanemaaijer E, Martier R, et al. Design, characterization, and lead selection of therapeutic miRNAs targeting huntingtin for development of gene therapy for Huntington’s disease. Mol Ther Nucleic Acids. 2016;5:e297.
Sibley CR, Wood MJA. Identification of allele-specific RNAi effectors targeting genetic forms of Parkinson’s disease. PLoS ONE. 2011;6:e26194.
Morelli KH, Griffin LB, Pyne NK, Wallace LM, Fowler AM, Oprescu SN, et al. Allele-specific RNA interference prevents neuropathy in Charcot-Marie-Tooth disease type 2D mouse models. J Clin Invest. 2019;129:5568–83.
Wallace LM, Saad NY, Pyne NK, Fowler AM, Eidahl JO, Domire JS, et al. Pre-clinical safety and off-target studies to support translation of AAV-mediated RNAi therapy for FSHD. Mol Ther Methods Clin Dev. 2018;8:121–30.
Li X, Sun X, Kan C, Chen B, Qu N, Hou N, et al. COL1A1: A novel oncogenic gene and therapeutic target in malignancies. Pathol Res Pract. 2022;236:154013.
Vega F, Medeiros LJ, Bueso-Ramos CE, Arboleda P, Miranda RN. Hematolymphoid neoplasms associated with rearrangements of PDGFRA, PDGFRB, and FGFR1. Am J Clin Pathol. 2015;144:377–92.
Acknowledgements
We thank the Cloning Facility at Marlin Biotech LLС (Marina A. Dzhenkova, Anna Karan) for creating plasmids and the Viral Vector Core (Anna A. Shmidt, Alexandra Y. Khamatova, Marina A. Dzhenkova, Vyacheslav A. Loginov, Daria V. Tsvirkun) for the AAV production. We thank the Center for Precision Genome Editing and Genetic Technologies for Biomedicine (Institute of Gene Biology RAS) for providing the equipment and Anna V. Tvorogova for assistance with confocal imaging. We acknowledge the input of Evgenia D. Zotova and Alisa V. Muraveva in the RNA-seq experiment.
Funding
The work on creating control AAV-miR vectors and characterization of patient-specific neurons was supported by the Russian Science Foundation, project 23-25-00323.
Author information
Authors and Affiliations
Contributions
MVB developed concept; MVB, TVE, AR, and AVD supervised the study; EAL performed protein functional characterization; DJ, VGK, MS, EAL, and AR performed the electrophysiology study; MVB, NVK, EAS, EAL, and MYS developed the AAV-RNAi approach; IMS performed qPCR analysis; EAV and DMS provided patient’s neurons; MVU and SP performed RNA-seq; EAL and KSA analyzed RNA-seq data; SGV, AVP, VMP, PRL, and AVD performed the mouse safety study. MVB, NVK, EAL, and AR wrote the original manuscript; TVE, KSA, and MYS reviewed and provided critiques; DJ and SP made corrections to the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
Animal studies were carried out in compliance with Directive 2010/63/EU; experimental protocols were approved by the local Ethics Committee of the Institute of Gene Biology and Belgorod State National Research University.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lunev, E.A., Klementieva, N.V., Vassilieva, S.G. et al. Development of an AAV-RNAi strategy to silence the dominant variant GNAO1 c.607G>A linked to encephalopathy. Gene Ther (2025). https://doi.org/10.1038/s41434-025-00532-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41434-025-00532-x