Abstract
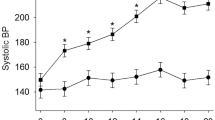
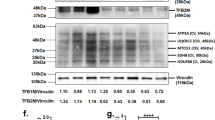
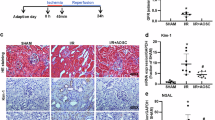
We performed a DNA microarray analysis of the renal medulla and cortex from spontaneously hypertensive rats (SHRs), stroke-prone SHRs (SHRSPs), and Wistar–Kyoto (WKY) rats to identify pivotal molecules in the kidney associated with the onset of hypertension and found increased expression of acyl-CoA oxidase 2 (Acox2) mRNA. Real-time polymerase chain reaction revealed that Acox2 mRNA expression in the renal medulla and cortex of SHRs and SHRSPs was increased in comparison to WKY rats. These findings indicate that increased renal ACOX2 (an enzyme that induces the β-oxidation of fatty acids) is associated with the onset of hypertension. Immunostaining of ACOX2 in the distal tubules from SHRs was stronger than that in the distal tubules from WKY rats. Western blot analysis showed increased expression of ACOX2 protein in renal medulla from SHRs. Regarding the overexpression of ACOX2, plasma levels of phytanic acid in SHRs were significantly higher than those in WKY rats. There were no differences in other short-chain fatty acids. Plasma phytanic acid was affected by the gut microbiota through the conversion from phytol by yeast in the intestinal tract. We compared the gut microbiota profile in three strains of 5-week-old rats by the terminal-restriction fragment length polymorphism method. The gut microbiota profile and ratio of Firmicutes/Bacteroides differed between SHRs and WKY rats. These findings suggest that the increased expression of ACOX2 in the kidney along with increases in plasma phytanic acid and the altered gut microbiota may be involved in the oxidation in the kidney and the pathogenesis of hypertension.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Rettig R, Folberth C, Stauss H, Kopf D, Waldherr R, Unger T. Role of the kidney in primary hypertension: a renal transplantation study in rats. Am J Physiol. 1990;258:F606–11.
Patschan O, Kuttler B, Heeman U, Uber A, Rettig R. Kidneys from normotensive donors lower blood pressure in young transplanted spontaneously hypertensive rats. Am J Physiol. 1997;273:R175–80.
Pavlov TS, Staruschenko A. Involvement of ENaC in the development of salt-sensitive hypertension. Am J Physiol Ren Physiol. 2017;313:F135–40.
Okamoto K, Yamori Y, Nagaoka Y. Establishment of the stroke-prone spontaneously hypertensive rats. Circ Res. 1974;34:143–53.
Nakaya H, Sasamura H, Kitamura Y, Amemiya T, Konishi K, Hayashi M, et al. Effects of angiotensin inhibitors on renal injury and angiotensin receptor expression in early hypertensive nephrosclerosis. Hypertens Res. 1999;22:303–12.
Obata J, Nakamura T, Takano H, Naito A, Kimura H, Yoshida Y, et al. Increased gene expression of components of the renin-angiotensin system in glomeruli of genetically hypertensive rats. J Hypertens. 2000;18:1247–55.
Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, et al. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004;44:1–6.
Negishi E, Fukuda N, Otsuki T, Katakawa M, Komatsu K, Chen L, et al. Involvement of complement 3 in the salt-sensitive hypertension by activation of renal renin-angiotensin system in spontaneously hypertensive rats. Am J Physiol Ren Physiol. 2018;315:F1747–58.
Chen L, Fukuda N, Matsumoto T, Abe M. Role of complement 3 in the pathogenesis of hypertension. Hypertens Res. 2020;43:255–62.
Hultström M. Development of structural kidney damage in spontaneously hypertensive rats. J Hypertens. 2012;30:1087–91.
Biswas SK, de Faria JB. Which comes first: renal inflammation or oxidative stress in spontaneously hypertensive rats? Free Radic Res. 2007;41:216–24.
Zhan CD, Sindhu RK, Vaziri ND. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: reversal by lifelong antioxidant supplementation. Kidney Int. 2004;65:219–27.
Miyamoto A, Aoyama T, Okamura M, Fukuda N, Ueno T, Abe M, et al. Development of a method for measuring phytanic acid as a lifestyle-related disease biomarker in rat serum using ultra-fast liquid chromatography-ultraviolet spectrophotometry combined with a modified 2-nitrophenylhydrazine derivatization method. Anal Sci. 2017;33:365–8.
Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Investig. 1985;75:1448–54.
Nagashima K, Sawa S, Nitta T, Tsutsumi M, Okamura T, Penninger JM, et al. Identification of subepithelial mesenchymal cells that induce IgA and diversify gut microbiota. Nat Immunol. 2017;18:675–82.
Hariya N, Miyake K, Kubota T, Goda T, Mochizuki K. Putative PPAR target genes express highly in skeletal muscle of insulin-resistant MetS model SHR/NDmc-cp rats. J Nutr Sci Vitaminol. 2015;61:28–36.
Skrede S, Sørensen HN, Larsen LN, Steineger HH, Høvik K, Spydevold OS, et al. Thia fatty acids, metabolism and metabolic effects. Biochim Biophys Acta. 1997;1344:115–31.
Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Science. 2005;308:1635–8.
Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;63:1–10.
Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature. 2006;444:1022–3.
Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, et al. Olfactory receptor responding to gut microbiota derived signals plays a role in renin secretion and blood pressure regulation. PNAS. 2013;110:4410–5.
Morris DJ, Ridlon JM. Glucocorticoids and gut bacteria: “The GALF Hypothesis” in the metagenomic era. Steroids. 2017;125:1–13.
Honour JW, Borriello SP, Ganten U, Honour P. Antibiotics attenuate experimental hypertension in rats. J Endocrinol. 1985;105:347–50.
Honour JW. Historical perspective: gut dysbiosis and hypertension. Physiol Genom. 2015;47:443–6.
Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40.
Chen D, Xiao C, Jin H, Yang B, Niu J, Yan S, et al. Exposure to atmospheric pollutants is associated with alterations of gut microbiota in spontaneously hypertensive rats. Exp Ther Med. 2019;18:3484–92.
Hellgren LI. Phytanic acid–an overlooked bioactive fatty acid in dairy fat? Ann N Y Acad Sci. 2010;1190:42–49.
Brita NC, Oksbjerg N, Hellgren LI, Nielsen JH, Young JF. Phytanic acid stimulates glucose uptake in a model of skeletal muscles, the primary porcine myotubes. Lipids Health Dis. 2013;12:14.
Roca-Saavedra P, Marino-Lorenzo P, Miranda JM, Porto-Aris JJ, Vazquez BI, Franco CM, et al. Phytanic acid comsumption and hyman health, risks, benefits and future trends: a review. Food Chem. 2017;221:137–247.
Oyama J, Node K. Gut microbiota and hypertension. Hypertens Res. 2019;42:741–3.
Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–77.
de la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2018;11:E51.
Konukoglu UzunH. Endothelial dysfunction and hypertension. Adv Exp Med Biol. 2017;956:511–40.
Wilcox CS. Redox regulation of the afferent arteriole and tubuloglomerular feedback. Acta Physiol Scand. 2003;179:217–23.
Welch WJ, Tojo A, Wilcox CS. Roles of NO and oxygen radicals in tubuloglomerular feedback in SHR. Am J Physiol. 2000;278:F769–76.
Duni A, Liakopoulos V, Roumeliotis S, Peschos D, Dounousi E. Oxidative stress in the pathogenesis and evolution of chronic kidney disease: untangling Ariadne’s thread. Int J Mol Sci. 2019;20:E3711.
Acknowledgements
The authors would like to thank Akiko Tsunemi and Mayumi Katakawa for their technical support. The authors acknowledge the support of this study by financial grants from the MEXT-Supported Program for the Strategic Research Foundation at Private Universities (S1411018).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Okamura, M., Ueno, T., Tanaka, S. et al. Increased expression of acyl-CoA oxidase 2 in the kidney with plasma phytanic acid and altered gut microbiota in spontaneously hypertensive rats. Hypertens Res 44, 651–661 (2021). https://doi.org/10.1038/s41440-020-00611-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-020-00611-z