Abstract
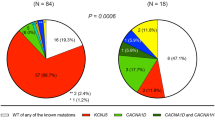
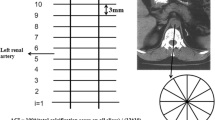
Autonomous cortisol secretion (ACS) is not uncommon in patients with primary aldosteronism (PA). However, the cardiovascular burden of ACS due to its dysregulated cortisol secretion remains poorly understood. Thus, we examined the effects of ACS on vascular calcification in a hyperaldosteronism environment in vitro and in vivo. A total of 339 patients with PA with adrenal incidentaloma and low-dose dexamethasone suppression test data (cutoff level: cortisol > 1.8 μg/dL) from a prospectively maintained database were enrolled; abdominal aortic calcification (AAC) scores were quantitatively estimated. Human aortic smooth muscle cells (HAOSMCs) were used as in vitro model of vascular calcification. In this study, 65 of the 339 patients with PA had ACS; 274 did not. Patients with PA/ACS had a higher AAC score (1171.0 ± 2434.0 vs. 489.5 ± 1085.3, P = 0.012) than patients without ACS. ACS was independently associated with AAC score (β = 0.139, P = 0.004) in multivariate analysis, and post-suppression cortisol level was significantly correlated with the AAC score (P = 0.004). In the HAOSMC model, co-treatment with cortisol synergistically stimulated alkaline phosphatase activity and calcium deposition in a hyperaldosteronism environment. The stimulatory effect of cortisol was abolished by the mineralocorticoid receptor (MR) antagonist eplerenone, but not glucocorticoid receptor antagonist mifepristone, indicating a MR-dependent mechanism. In conclusion, the presence of ACS is associated with heavier vascular calcification in patients with PA. Aldosterone and cortisol synergistically activate HAOSMC calcification via MR signaling, via a process that can be attenuated by eplerenone.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Rossi GP, Bernini G, Caliumi C, Desideri G, Fabris B, Ferri C, et al. A prospective study of the prevalence of primary aldosteronism in 1,125 hypertensive patients. J Am Coll Cardiol. 2006;48:2293–300.
Hiraishi K, Yoshimoto T, Tsuchiya K, Minami I, Doi M, Izumiyama H, et al. Clinicopathological features of primary aldosteronism associated with subclinical Cushing’s syndrome. Endocr J. 2011;58:543–51.
Tang L, Li X, Wang B, Ma X, Li H, Gao Y, et al. Clinical characteristics of aldosterone- and cortisol-coproducing adrenal adenoma in primary aldosteronism. Int J Endocrinol. 2018;2018:4920841.
Piaditis GP, Kaltsas GA, Androulakis II, Gouli A, Makras P, Papadogias D, et al. High prevalence of autonomous cortisol and aldosterone secretion from adrenal adenomas. Clin Endocrinol. 2009;71:772–8.
Peng KY, Liao HW, Chan CK, Lin WC, Yang SY, Tsai YC, et al. Presence of subclinical hypercortisolism in clinical aldosterone-producing adenomas predicts lower clinical success. Hypertension. 2020;76:1537–44.
Terzolo M, Pia A, Reimondo G. Subclinical Cushing’s syndrome: definition and management. Clin Endocrinol. 2012;76:12–8.
Bancos I, Alahdab F, Crowley RK, Chortis V, Delivanis DA, Erickson D, et al. THERAPY OF ENDOCRINE DISEASE: improvement of cardiovascular risk factors after adrenalectomy in patients with adrenal tumors and subclinical Cushing’s syndrome: a systematic review and meta-analysis. Eur J Endocrinol. 2016;175:R283–95.
Yasuda S, Hikima Y, Kabeya Y, Iida S, Oikawa Y, Isshiki M, et al. Clinical characterization of patients with primary aldosteronism plus subclinical Cushing’s syndrome. BMC Endocr Disord. 2020;20:9.
Araujo-Castro M, Pascual-Corrales E, Lamas C. Possible, probable, and certain hypercortisolism: a continuum in the risk of comorbidity. Ann Endocrinol (Paris). 2023;84:272–84.
Di Dalmazi G, Vicennati V, Garelli S, Casadio E, Rinaldi E, Giampalma E, et al. Cardiovascular events and mortality in patients with adrenal incidentalomas that are either non-secreting or associated with intermediate phenotype or subclinical Cushing’s syndrome: a 15-year retrospective study. Lancet Diabetes Endocrinol. 2014;2:396–405.
Farman N, Bocchi B. Mineralocorticoid selectivity: molecular and cellular aspects. Kidney Int. 2000;57:1364–9.
Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–70.
Bostrom K, Watson KE, Stanford WP, Demer LL. Atherosclerotic calcification: relation to developmental osteogenesis. Am J Cardiol. 1995;75:88B–91B.
O’Connor SD, Graffy PM, Zea R, Pickhardt PJ. Does nonenhanced CT-based quantification of abdominal aortic calcification outperform the framingham risk score in predicting cardiovascular events in asymptomatic adults? Radiology. 2019;290:108–15.
Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, et al. Case detection and diagnosis of primary aldosteronism—the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005.
Wu VC, Yang SY, Lin JW, Cheng BW, Kuo CC, Tsai CT, et al. Kidney impairment in primary aldosteronism. Clin Chim Acta. 2011;412:1319–25.
Fassnacht M, Arlt W, Bancos I, Dralle H, Newell-Price J, Sahdev A, et al. Management of adrenal incidentalomas: European society of endocrinology clinical practice guideline in collaboration with the European network for the study of adrenal tumors. Eur J Endocrinol. 2016;175:G1–34.
Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, et al. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2008;93:1526–40.
Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–32.
Zhu CJ, Wang QQ, Zhou JL, Liu HZ, Hua F, Yang HZ, et al. The mineralocorticoid receptor-p38MAPK-NFkappaB or ERK-Sp1 signal pathways mediate aldosterone-stimulated inflammatory and profibrotic responses in rat vascular smooth muscle cells. Acta Pharm Sin. 2012;33:873–8.
Ishizawa K, Izawa Y, Ito H, Miki C, Miyata K, Fujita Y, et al. Aldosterone stimulates vascular smooth muscle cell proliferation via big mitogen-activated protein kinase 1 activation. Hypertension. 2005;46:1046–52.
Jaffe IZ, Tintut Y, Newfell BG, Demer LL, Mendelsohn ME. Mineralocorticoid receptor activation promotes vascular cell calcification. Arterioscler Thromb Vasc Biol. 2007;27:799–805.
Peng SY, Tsai CH, Wu XM, Huang HH, Chen ZW, Lee BC, et al. Aldosterone suppresses endothelial mitochondria through mineralocorticoid receptor/mitochondrial reactive oxygen species pathway. Biomedicines. 2022;10:1119.
Bonewald LF, Harris SE, Rosser J, Dallas MR, Dallas SL, Camacho NP, et al. von Kossa staining alone is not sufficient to confirm that mineralization in vitro represents bone formation. Calcif Tissue Int. 2003;72:537–47.
Desquilbet L, Mariotti F. Dose-response analyses using restricted cubic spline functions in public health research. Stat Med. 2010;29:1037–57.
Nakajima Y, Yamada M, Taguchi R, Satoh T, Hashimoto K, Ozawa A, et al. Cardiovascular complications of patients with aldosteronism associated with autonomous cortisol secretion. J Clin Endocrinol Metab. 2011;96:2512–8.
Erem C, Nuhoglu I, Yilmaz M, Kocak M, Demirel A, Ucuncu O, et al. Blood coagulation and fibrinolysis in patients with Cushing’s syndrome: increased plasminogen activator inhibitor-1, decreased tissue factor pathway inhibitor, and unchanged thrombin-activatable fibrinolysis inhibitor levels. J Endocrinol Invest. 2009;32:169–74.
Zacharieva S, Atanassova I, Orbetzova M, Kirilov G, Nachev E, Kalinov K, et al. Vascular endothelial growth factor (VEGF), prostaglandin E2(PGE2) and active renin in hypertension of adrenal origin. J Endocrinol Invest. 2004;27:742–6.
Rudelli S, Viriato SP, Meireles TL, Frederico TN. Treatment of displaced neck fractures of the femur with total hip arthroplasty. J Arthroplast. 2012;27:246–52.
Neary NM, Booker OJ, Abel BS, Matta JR, Muldoon N, Sinaii N, et al. Hypercortisolism is associated with increased coronary arterial atherosclerosis: analysis of noninvasive coronary angiography using multidetector computerized tomography. J Clin Endocrinol Metab. 2013;98:2045–52.
Viengchareun S, Le Menuet D, Martinerie L, Munier M, Pascual-Le Tallec L, Lombes M. The mineralocorticoid receptor: insights into its molecular and (patho)physiological biology. Nucl Recept Signal. 2007;5:e012.
Gomez-Sanchez E, Gomez-Sanchez CE. The multifaceted mineralocorticoid receptor. Compr Physiol. 2014;4:965–94.
Song IH, Buttgereit F. Non-genomic glucocorticoid effects to provide the basis for new drug developments. Mol Cell Endocrinol. 2006;246:142–6.
Longenecker JP, Kilty LA, Johnson LK. Glucocorticoid inhibition of vascular smooth muscle cell proliferation: influence of homologous extracellular matrix and serum mitogens. J Cell Biol. 1984;98:534–40.
Son BK, Akishita M, Iijima K, Eto M, Ouchi Y. Mechanism of pi-induced vascular calcification. J Atheroscler Thromb. 2008;15:63–68.
Pustlauk W, Westhoff TH, Claeys L, Roch T, Geissler S, Babel N. Induced osteogenic differentiation of human smooth muscle cells as a model of vascular calcification. Sci Rep. 2020;10:5951.
Jaffe IZ, Mendelsohn ME. Angiotensin II and aldosterone regulate gene transcription via functional mineralocortocoid receptors in human coronary artery smooth muscle cells. Circ Res. 2005;96:643–50.
Haarhaus M, Arnqvist HJ, Magnusson P. Calcifying human aortic smooth muscle cells express different bone alkaline phosphatase isoforms, including the novel B1x isoform. J Vasc Res. 2013;50:167–74.
Luong TTD, Estepa M, Boehme B, Pieske B, Lang F, Eckardt KU, et al. Inhibition of vascular smooth muscle cell calcification by vasorin through interference with TGFbeta1 signaling. Cell Signal. 2019;64:109414.
Jeong J, Cho S, Seo M, Lee BS, Jang Y, Lim S, et al. Soluble RAGE attenuates Ang II-induced arterial calcification via inhibiting AT1R-HMGB1-RAGE axis. Atherosclerosis. 2022;346:53–62.
van de Wal RM, Plokker HW, Lok DJ, Boomsma F, van der Horst FA, van Veldhuisen DJ, et al. Determinants of increased angiotensin II levels in severe chronic heart failure patients despite ACE inhibition. Int J Cardiol. 2006;106:367–72.
Amano T, Matsubara T, Izawa H, Torigoe M, Yoshida T, Hamaguchi Y, et al. Impact of plasma aldosterone levels for prediction of in-stent restenosis. Am J Cardiol. 2006;97:785–8.
Tsai CH, Liao CW, Wu XM, Chen ZW, Pan CT, Chang YY, et al. Autonomous cortisol secretion is associated with worse arterial stiffness and vascular fibrosis in primary aldosteronism: a cross-sectional study with follow-up data. Eur J Endocrinol. 2022;187:197–208.
Adolf C, Kohler A, Franke A, Lang K, Riester A, Low A, et al. Cortisol excess in patients with primary aldosteronism impacts left ventricular hypertrophy. J Clin Endocrinol Metab. 2018;103:4543–52.
Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–75.
Mihailidou AS, Loan Le TY, Mardini M, Funder JW. Glucocorticoids activate cardiac mineralocorticoid receptors during experimental myocardial infarction. Hypertension. 2009;54:1306–12.
Bastos Goncalves F, Voute MT, Hoeks SE, Chonchol MB, Boersma EE, Stolker RJ, et al. Calcification of the abdominal aorta as an independent predictor of cardiovascular events: a meta-analysis. Heart. 2012;98:988–94.
Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019;140:e563–95.
Allen SL, Elliott BT, Carson BP, Breen L. Improving physiological relevance of cell culture: the possibilities, considerations, and future directions of the ex vivo coculture model. Am J Physiol Cell Physiol. 2023;324:C420–7.
The TAIPAI study group
Vin-Cent Wu12, Tai-Shuan Lai12, Shih-Chieh Jeff Chueh12, Shao-Yu Yang12, Kao-Lang Liu12, Chin-Chen Chang12, Bo-Ching Lee12, Shuo-Meng Wang12, Kuo-How Huang12, Po-Chih Lin12, Yen-Hung Lin12, Chi-Sheng Hung12, Lian-Yu Lin12, Shih-Cheng Liao12, Ching-Chu Lu12, Chieh-Kai Chan12, Leay-Kiaw Er13, Ya-Hui Hu13, Che-Hsiung Wu13, Yao-Chou Tsai13, Zheng-Wei Chen14, Chien-Ting Pan14, Che-Wei Liao15, Cheng-Hsuan Tsai12, Yi-Yao Chang16, Chen-Hsun Ho17, Wei-Chieh Huang18, Ying-Ying Chen19.
Funding
This study was supported by National Science and Technology Council (111-2314-B-002-250-MY2). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Consortia
Contributions
Bo-Ching Lee project concept and design, data collection, imaging analysis, data analysis, and write-up. Victor Jing-Wei Kang data collection, imaging analysis, write-up. Chin-Chen Chang project concept and design. Jia-Zheng Huang data collection, imaging analysis. Yi-Yao Chang project concept and design. Cheng-Hsuan Tsai project concept and design. Zheng-Wei Chen project concept and design. Yu-Li Lin project concept and design. Chia-Hung Chou critical revisions. Che-Wei Liao critical revisions. Chien-Ting Pan critical revisions. Chi-Sheng Hung critical revisions. Vin-Cent Wu project concept and design, data collection, critical revisions. Yen-Hung Lin project concept and design, data collection, imaging analysis, critical revisions.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lee, BC., Chang, CC., Kang, V.JW. et al. Autonomous cortisol secretion promotes vascular calcification in vivo and in vitro under hyperaldosteronism. Hypertens Res 48, 366–377 (2025). https://doi.org/10.1038/s41440-024-01935-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01935-w
Keywords
This article is cited by
-
Synergistic interplay between cortisol and aldosterone: unveiling mechanisms of vascular calcification in hyperaldosteronism
Hypertension Research (2025)
-
Exploring the high prevalence, comorbidities, and indicators of mild autonomous cortisol secretion in primary aldosteronism: a cohort study and systematic review
Hypertension Research (2025)