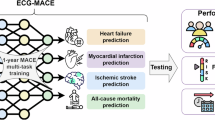
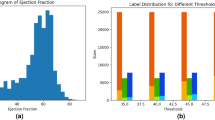
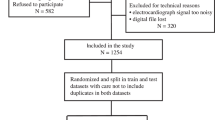
Abstract
Arterial hypertension is a major risk factor for cardiovascular diseases. While cardiac ultrasound is a typical way to diagnose hypertension-mediated heart change, it often fails to detect early subtle structural changes. Electrocardiogram(ECG) represents electrical activity of heart muscle, affected by the changes in heart’s structure. It is crucial to explore whether ECG can capture slight signals of hypertension-mediated heart change. However, reading ECG records is complex and some signals are too subtle to be captured by cardiologist’s visual inspection. In this study, we designed a deep learning model to predict hypertension on ECG signals and then to identify hypertension-associated ECG segments. From The First Affiliated Hospital of Xiamen University, we collected 210,120 10-s 12-lead ECGs using the FX-8322 manufactured by FUKUDA and 812 ECGs using the RAGE-12 manufactured by NALONG. We proposed a deep learning framework, including MML-Net, a multi-branch, multi-scale LSTM neural network to evaluate the potential of ECG signals to detect hypertension, and ECG-XAI, an ECG-oriented wave-alignment AI explanation pipeline to identify hypertension-associated ECG segments. MML-Net achieved an 82% recall and an 87% precision in the testing, and an 80% recall and an 82% precision in the independent testing. In contrast, experienced clinical cardiologists typically attain recall rates ranging from 30 to 50% by visual inspection. The experiments demonstrate that ECG signals are sensitive to slight changes in heart structure caused by hypertension. ECG-XAI detects that R-wave and P-wave are the hypertension-associated ECG segments. The proposed framework has the potential to facilitate early diagnosis of heart change.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
16 December 2024
A Correction to this paper has been published: https://doi.org/10.1038/s41440-024-01980-5
References
Dzau VJ, Balatbat CA. Future of hypertension the need for transformation. Hypertension. 2019;74:450–7. https://doi.org/10.1161/Hypertensionaha.119.13437
Desai AN. High blood pressure. JAMA. 2020;324:1254–5. https://doi.org/10.1001/jama.2020.11289
Fuchs FD, Whelton PK. High blood pressure and cardiovascular disease. Hypertension. 2020;75:285–92. https://doi.org/10.1161/HYPERTENSIONAHA.119.14240
Dzeshka MS, Shantsila A, Shantsila E, Lip GYH. Atrial fibrillation and hypertension. Hypertension. 2017;70:854–61. https://doi.org/10.1161/HYPERTENSIONAHA.117.08934
Yildiz M, Oktay AA, Stewart MH, Milani RV, Ventura HO, Lavie CJ. Left ventricular hypertrophy and hypertension. Prog Cardiovascular Dis. 2020;63:10–21. https://doi.org/10.1016/j.pcad.2019.11.009
Velagaleti RS, Gona P, Pencina MJ, Aragam J, Wang TJ, Levy D, et al. Left ventricular hypertrophy patterns and incidence of heart failure with preserved versus reduced ejection fraction. Am J Cardiol. 2014;113:117–22. https://doi.org/10.1016/j.amjcard.2013.09.028
Valente AM, Lakdawala NK, Powell AJ, Evans SP, Cirino AL, Orav EJ, et al. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ Cardiovasc Genet. 2013;6:230–7. https://doi.org/10.1161/CIRCGENETICS.113.000037
Oseni AO, Qureshi WT, Almahmoud MF, Bertoni AG, Bluemke DA, Hundley WG, et al. Left ventricular hypertrophy by ECG versus cardiac MRI as a predictor for heart failure. Heart. 2017;103:49–54. https://doi.org/10.1136/heartjnl-2016-309516
Derumeaux G, Mulder P, Richard V, Chagraoui A, Nafeh C, Bauer F, et al. Tissue Doppler imaging differentiates physiological from pathological pressure-overload left ventricular hypertrophy in rats. Circulation. 2002;105:1602–8. https://doi.org/10.1161/01.cir.0000012943.91101.d7
Bacharova L. ECG in left ventricular hypertrophy: A change in paradigm from assessing left ventricular mass to its electrophysiological properties. J Electrocardiol. 2022;73:153–6. https://doi.org/10.1016/j.jelectrocard.2022.06.002
Boles U, Enriquez A, Ghabra WA, Abdollah H, Michael KA. Early changes on the electrocardiogram in hypertension. e-journal of the ESC Council for Cardiology Practice. 2015;13:30.
Miceli F, Presta V, Citoni B, Canichella F, Figliuzzi I, Ferrucci A, et al. Conventional and new electrocardiographic criteria for hypertension-mediated cardiac organ damage: A narrative review. J Clin Hypertens (Greenwich). 2019;21:1863–71. https://doi.org/10.1111/jch.13726
De la Garza Salazar F, Romero Ibarguengoitia ME, Azpiri Lopez JR, Gonzalez Cantu A. Optimizing ECG to detect echocardiographic left ventricular hypertrophy with computer-based ECG data and machine learning. PLoS One. 2021;16:e0260661 https://doi.org/10.1371/journal.pone.0260661
Su F-Y, Li Y-H, Lin Y-P, Lee C-J, Wang C-H, Meng F-C, et al. A comparison of Cornell and Sokolow-Lyon electrocardiographic criteria for left ventricular hypertrophy in a military male population in Taiwan: the Cardiorespiratory fitness and HospItalization Events in armed Forces study. Cardiovascular Diagn Ther. 2017;7:244–51.
Levy D, Labib SB, Anderson KM, Christiansen JC, Kannel WB, Castelli WP. Determinants of sensitivity and specificity of electrocardiographic criteria for left ventricular hypertrophy. Circulation. 1990;81:815–20. https://doi.org/10.1161/01.cir.81.3.815
Molloy TJ, Okin PM, Devereux RB, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy by the simple QRS voltage-duration product. J Am Coll Cardiol. 1992;20:1180–6. https://doi.org/10.1016/0735-1097(92)90376-x
Somani S, Russak AJ, Richter F, Zhao S, Vaid A, Chaudhry F, et al. Deep learning and the electrocardiogram: review of the current state-of-the-art. Europace. 2021;23:1179–91. https://doi.org/10.1093/europace/euaa377
Kwon JM, Lee SY, Jeon KH, Lee Y, Kim KH, Park J, et al. Deep learning-based algorithm for detecting aortic stenosis using electrocardiography. J Am Heart Assoc. 2020;9:e014717 https://doi.org/10.1161/JAHA.119.014717
Kwon JM, Jeon KH, Kim HM, Kim MJ, Lim SM, Kim KH, et al. Comparing the performance of artificial intelligence and conventional diagnosis criteria for detecting left ventricular hypertrophy using electrocardiography. Europace. 2020;22:412–9. https://doi.org/10.1093/europace/euz324
Cai W, Chen Y, Guo J, Han B, Shi Y, Ji L, et al. Accurate detection of atrial fibrillation from 12-lead ECG using deep neural network. Comput Biol Med. 2020;116:103378 https://doi.org/10.1016/j.compbiomed.2019.103378
Ko WY, Siontis KC, Attia ZI, Carter RE, Kapa S, Ommen SR, et al. Detection of hypertrophic cardiomyopathy using a convolutional neural network-enabled electrocardiogram. J Am Coll Cardiol. 2020;75:722–33. https://doi.org/10.1016/j.jacc.2019.12.030
Goto S, Solanki D, John JE, Yagi R, Homilius M, Ichihara G, et al. Multinational federated learning approach to train ECG and echocardiogram models for hypertrophic cardiomyopathy detection. Circulation. 2022;146:755–69. https://doi.org/10.1161/CIRCULATIONAHA.121.058696
Hu J, Shen L, Sun G. Squeeze-and-excitation networks. Paper/Poster presented at: Proceedings of the IEEE conference on computer vision and pattern recognition; 2018;
Makowski D, Pham T, Lau ZJ, Brammer JC, Lespinasse F, Pham H, et al. NeuroKit2: a python toolbox for neurophysiological signal processing. Behav Res Methods. 2021;53:1689–96. https://doi.org/10.3758/s13428-020-01516-y
Jiang P-T, Zhang C-B, Hou Q, Cheng M-M, Wei Y. LayerCAM: exploring hierarchical class activation maps for localization. IEEE Transations Image Process. 2021;30:5875–88. https://doi.org/10.1109/TIP.2021.3089943.
Sakli N, Ghabri H, Soufiene BO, Almalki FA, Sakli H, Ali O, et al. ResNet-50 for 12-lead electrocardiogram automated diagnosis. Comput Intell Neurosci. 2022;2022:7617551 https://doi.org/10.1155/2022/7617551
Murugesan B, Ravichandran V, Ram K, P SP, Joseph J, Shankaranarayana SM, et al. ECGNet: Deep Network for Arrhythmia Classification. Paper/Poster presented at: 2018 IEEE International Symposium on Medical Measurements and Applications (MeMeA); 11-13 2018, 2018;
Kropf M, Hayn D, Schreier G. ECG classification based on time and frequency ___domain features using random forests. Paper/Poster presented at: 2017 Computing in Cardiology (CinC); 24-27 Sept. 2017, 2017;
Ryu SY, Lee SH, Isenberg G, Ho WK, Earm YE. Monitoring of ANP secretion from single atrial myocytes using densitometry. Pflug Arch. 2002;444:568–77. https://doi.org/10.1007/s00424-002-0852-7
Mayyas F, Niebauer M, Zurick A, Barnard J, Gillinov AM, Chung MK, et al. Association of left atrial endothelin-1 with atrial rhythm, size, and fibrosis in patients with structural heart disease. Circ Arrhythm Electrophysiol. 2010;3:369–79. https://doi.org/10.1161/CIRCEP.109.924985
Triposkiadis F, Pieske B, Butler J, Parissis J, Giamouzis G, Skoularigis J, et al. Global left atrial failure in heart failure. Eur J Heart Fail. 2016;18:1307–20. https://doi.org/10.1002/ejhf.645
Lau YF, Yiu KH, Siu CW, Tse HF. Hypertension and atrial fibrillation: epidemiology, pathophysiology and therapeutic implications. J Hum Hypertens. 2012;26:563–9. https://doi.org/10.1038/jhh.2011.105
Noresson E, Ricksten SE, Thoren P. Left atrial pressure in normotensive and spontaneously hypertensive rats. Acta Physiol Scand. 1979;107:9–12. https://doi.org/10.1111/j.1748-1716.1979.tb06436.x
Choisy SC, Arberry LA, Hancox JC, James AF. Increased susceptibility to atrial tachyarrhythmia in spontaneously hypertensive rat hearts. Hypertension. 2007;49:498–505. https://doi.org/10.1161/01.HYP.0000257123.95372.ab
Kistler PM, Sanders P, Dodic M, Spence SJ, Samuel CS, Zhao C, et al. Atrial electrical and structural abnormalities in an ovine model of chronic blood pressure elevation after prenatal corticosteroid exposure: implications for development of atrial fibrillation. Eur Heart J. 2006;27:3045–56. https://doi.org/10.1093/eurheartj/ehl360
Lau DH, Mackenzie L, Kelly DJ, Psaltis PJ, Worthington M, Rajendram A, et al. Short-term hypertension is associated with the development of atrial fibrillation substrate: a study in an ovine hypertensive model. Heart Rhythm. 2010;7:396–404. https://doi.org/10.1016/j.hrthm.2009.11.031
Acknowledgements
Shaorong Fang and Tianfu Wu from Information and Network Center of Xiamen University are acknowledged for the help with high performance computing (HPC).
Funding
This work was supported by National Natural Science Foundation of China (62173282).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
The collected ECG records was approved by First Affiliated Hospital of Xiamen University Ethics Committee (reference 2023131).
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Fig. 1 in the original version of this article has been replaced. The original article has been corrected.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Liang, C., Yang, F., Huang, X. et al. Deep learning assists early-detection of hypertension-mediated heart change on ECG signals. Hypertens Res 48, 681–692 (2025). https://doi.org/10.1038/s41440-024-01938-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01938-7