Abstract
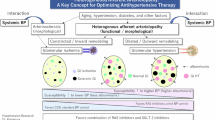
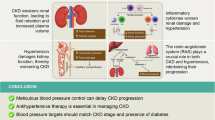
The primary treatment goal of chronic kidney disease (CKD) is preserving renal function and preventing its progression to end-stage renal disease. Glomerular hypertension and tubular hypoxia are critical risk factors in CKD progression. However, the renal hemodynamics make it difficult to avoid both factors due to the existence of peritubular capillaries that supply oxygen to the renal tubules downstream from the glomerulus through the efferent arteriole. In the treatment strategies for balancing glomerular pressure and tubular oxygen supply, afferent and efferent arterioles of the glomerulus determine glomerular filtration rate and blood flow to the peritubular capillaries. Therefore, sodium-glucose cotransporter 2 inhibitors and angiotensin receptor–neprilysin inhibitors as well as classical renin-angiotensin system inhibitors, which can change the diameter of afferent and/or efferent arterioles, are promising options for balancing this dual target and achieving renal protection. This review focuses on the clinical importance of glomerular pressure and tubular oxygen supply and proposes an effective treatment modality for this dual target.

This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Vallon V, Thomson SC. The tubular hypothesis of nephron filtration and diabetic kidney disease. Nat Rev Nephrol. 2020;16:317–36.
Nangaku M. Chronic hypoxia and tubulointerstitial injury: a final common pathway to end-stage renal failure. J Am Soc Nephrol. 2006;17:17–25.
Tanaka T, Miyata T, Inagi R, Fujita T, Nangaku M. Hypoxia in renal disease with proteinuria and/or glomerular hypertension. Am J Pathol. 2004;165:1979–92.
Kaneko S, Usui J, Takahashi K, Oda T, Yamagata K. Increased intrarenal post-glomerular blood flow is a key condition for the development of calcineurin inhibitor-induced renal tubular acidosis in kidney transplant recipients. Clin Transplant. 2022;36:e14648.
Azar S, Tobian L, Johnson MA. Glomerular, efferent arteriolar, peritubular capillary, and tubular pressures in hypertension. Am J Physiol. 1974;227:1045–50.
Vallon V. Renoprotective effects of SGLT2 inhibitors. Heart Fail Clin. 2022;18:539–49.
Haruhara K, Kanzaki G, Tsuboi N. Nephrons, podocytes and chronic kidney disease: strategic antihypertensive therapy for renoprotection. Hypertens Res. 2023;46:299–310.
Pelayo JC, Westcott JY. Impaired autoregulation of glomerular capillary hydrostatic pressure in the rat remnant nephron. J Clin Invest. 1991;88:101–5.
Nagata D, Hishida E, Masuda T. Practical strategy for treating chronic kidney disease (CKD)-associated with hypertension. Int J Nephrol Renov Dis. 2020;13:171–8.
Fujii M, Ohno Y, Ikeda A, Godai K, Li Y, Nakamura Y, et al. Current status of the rapid decline in renal function due to diabetes mellitus and its associated factors: analysis using the National Database of Health Checkups in Japan. Hypertens Res. 2023;46:1075–89.
Suenaga T, Satoh M, Murakami T, Hirose T, Obara T, Nakayama S, et al. Cross-classification by systolic and diastolic blood pressure levels and chronic kidney disease, proteinuria, or kidney function decline. Hypertens Res. 2023;46:1860–9.
Park CH, Jhee JH, Chun KH, Seo J, Lee CJ, Park SH, et al. Nocturnal systolic blood pressure dipping and progression of chronic kidney disease. Hypertens Res. 2024;47:215–24.
Haruhara K, Okabayashi Y, Sasaki T, Kubo E, D’Agati VD, Bertram JF, et al. Podocyte density as a predictor of long-term kidney outcome in obesity-related glomerulopathy. Kidney Int. 2024;106:496–507.
Denic A, Glassock RJ. Obesity-related glomerulopathy and single-nephron GFR. Kidney Int Rep. 2020;5:1126–8.
Okabayashi Y, Tsuboi N, Sasaki T, Haruhara K, Kanzaki G, Koike K, et al. Single-nephron GFR in patients with obesity-related glomerulopathy. Kidney Int Rep. 2020;5:1218–27.
Vallon V. How can inhibition of glucose and sodium transport in the early proximal tubule protect the cardiorenal system? Nephrol Dial Transplant. 2024;39:1565–73.
Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–40.
Evans RG, Gardiner BS, Smith DW, O’Connor PM. Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Ren Physiol. 2008;295:F1259–70.
Lankadeva YR, May CN, Bellomo R, Evans RG. Role of perioperative hypotension in postoperative acute kidney injury: a narrative review. Br J Anaesth. 2022;128:931–48.
Kurasawa S, Yasuda Y, Kato S, Maruyama S, Okada H, Kashihara N, et al. Relationship between the lower limit of systolic blood pressure target and kidney function decline in advanced chronic kidney disease: an instrumental variable analysis from the REACH-J CKD cohort study. Hypertens Res. 2023;46:2478–87.
Carlstrom M, Wilcox CS, Arendshorst WJ. Renal autoregulation in health and disease. Physiol Rev. 2015;95:405–511.
Pallone TL, Edwards A, Mattson DL. Renal medullary circulation. Compr Physiol. 2012;2:97–140.
Bohle A. Change of paradigms in nephrology—a view back and a look forward. Nephrol Dial Transplant. 1998;13:556–63.
Kang DH, Hughes J, Mazzali M, Schreiner GF, Johnson RJ. Impaired angiogenesis in the remnant kidney model: II. Vascular endothelial growth factor administration reduces renal fibrosis and stabilizes renal function. J Am Soc Nephrol. 2001;12:1448–57.
Ataga KI, Zhou Q, Derebail VK, Saraf SL, Hankins JS, Loehr LR, et al. Rapid decline in estimated glomerular filtration rate in sickle cell anemia: results of a multicenter pooled analysis. Haematologica 2021;106:1749–53.
Ohishi M. Hypertension with diabetes mellitus: physiology and pathology. Hypertens Res. 2018;41:389–93.
Yamamoto K, Rakugi H. Angiotensin receptor-neprilysin inhibitors: comprehensive review and implications in hypertension treatment. Hypertens Res. 2021;44:1239–50.
Nakayama T, Mitsuno R, Azegami T, Sato Y, Hayashi K, Itoh H. A systematic review and meta-analysis of the clinical impact of stopping renin-angiotensin system inhibitor in patients with chronic kidney disease. Hypertens Res. 2023;46:1525–35.
Hattori K, Sakaguchi Y, Oka T, Asahina Y, Kawaoka T, Doi Y, et al. Estimated effect of restarting renin-angiotensin system inhibitors after discontinuation on kidney outcomes and mortality. J Am Soc Nephrol. 2024;35:1391–401.
Tsukamoto S, Uehara T, Azushima K, Wakui H, Tamura K. Updates for cardio-kidney protective effects by angiotensin receptor-neprilysin inhibitor: requirement for additional evidence of kidney protection. J Am Heart Assoc. 2023;12:e029565.
Wang Y, Zhou R, Lu C, Chen Q, Xu T, Li D. Effects of the angiotensin-receptor neprilysin inhibitor on cardiac reverse remodeling: meta-analysis. J Am Heart Assoc. 2019;8:e012272.
McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004.
Williams B, Cockcroft JR, Kario K, Zappe DH, Brunel PC, Wang Q, et al. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension: the PARAMETER study. Hypertension. 2017;69:411–20.
Haynes R, Judge PK, Staplin N, Herrington WG, Storey BC, Bethel A, et al. Effects of sacubitril/valsartan versus irbesartan in patients with chronic kidney disease. Circulation. 2018;138:1505–14.
Theilig F, Wu Q. ANP-induced signaling cascade and its implications in renal pathophysiology. Am J Physiol Ren Physiol. 2015;308:F1047–1055.
Nishio H, Ishii A, Yamada H, Mori KP, Kato Y, Ohno S, et al. Sacubitril/valsartan ameliorates renal tubulointerstitial injury through increasing renal plasma flow in a mouse model of type 2 diabetes with aldosterone excess. Nephrol Dial Transplant. 2023;38:2517–27.
Vallon V. State-of-the-art-review mechanisms of action of SGLT2 inhibitors and clinical implications. Am J Hypertens 2024:hpae092. https://doi.org/10.1093/ajh/hpae092. Online ahead of print.
Mogi M, Tanaka A, Node K, Tomitani N, Hoshide S, Narita K, et al. 2023 update and perspectives. Hypertens Res. 2024;47:6–32.
Masuda T, Nagata D. Fluid homeostasis induced by sodium-glucose cotransporter 2 inhibitors: novel insight for better cardio-renal outcomes in chronic kidney disease. Hypertens Res. 2023;46:1195–201.
Masuda T, Yoshida M, Onaka T, Nagata D. Water and sodium conservation response induced by SGLT2 inhibitor ipragliflozin in Dahl salt-sensitive hypertensive rats. Hypertens Res. 2024. https://doi.org/10.1038/s41440-024-01893-3. Online ahead of print.
Thomson SC, Vallon V. Effects of SGLT2 inhibitor and dietary NaCl on glomerular hemodynamics assessed by micropuncture in diabetic rats. Am J Physiol Ren Physiol. 2021;320:F761–F771.
Wada Y, Kidokoro K, Kondo M, Tokuyama A, Kadoya H, Nagasu H, et al. Evaluation of glomerular hemodynamic changes by sodium-glucose-transporter 2 inhibition in type 2 diabetic rats using in vivo imaging. Kidney Int. 2024;106:408–18.
Rieg T, Masuda T, Gerasimova M, Mayoux E, Platt K, Powell DR, et al. Increase in SGLT1-mediated transport explains renal glucose reabsorption during genetic and pharmacological SGLT2 inhibition in euglycemia. Am J Physiol Ren Physiol. 2014;306:F188–93.
Onishi A, Fu Y, Patel R, Darshi M, Crespo-Masip M, Huang W, et al. A role for tubular Na+/H+ exchanger NHE3 in the natriuretic effect of the SGLT2 inhibitor empagliflozin. Am J Physiol Ren Physiol. 2020;319:F712–28.
Layton AT, Vallon V. SGLT2 inhibition in a kidney with reduced nephron number: modeling and analysis of solute transport and metabolism. Am J Physiol Ren Physiol. 2018;314:F969–84.
Gullaksen S, Vernstrom L, Sorensen SS, Ringgaard S, Laustsen C, Funck KL, et al. Separate and combined effects of semaglutide and empagliflozin on kidney oxygenation and perfusion in people with type 2 diabetes: a randomised trial. Diabetologia. 2023;66:813–25.
Ohara K, Masuda T, Morinari M, Okada M, Miki A, Nakagawa S, et al. The extracellular volume status predicts body fluid response to SGLT2 inhibitor dapagliflozin in diabetic kidney disease. Diabetol Metab Syndr. 2020;12:37.
Oka K, Masuda T, Ohara K, Miura M, Morinari M, Misawa K, et al. Fluid homeostatic action of dapagliflozin in patients with chronic kidney disease: the DAPA-BODY trial. Front Med. 2023;10:1287066.
Masuda T, Muto S, Fukuda K, Watanabe M, Ohara K, Koepsell H, et al. Osmotic diuresis by SGLT2 inhibition stimulates vasopressin-induced water reabsorption to maintain body fluid volume. Physiol Rep. 2020;8:e14360.
Masuda T, Ohara K, Vallon V, Nagata D. SGLT2 inhibitor and loop diuretic induce different vasopressin and fluid homeostatic responses in nondiabetic rats. Am J Physiol Ren Physiol. 2022;323:F361–9.
Masuda T, Watanabe Y, Fukuda K, Watanabe M, Onishi A, Ohara K, et al. Unmasking a sustained negative effect of SGLT2 inhibition on body fluid volume in the rat. Am J Physiol Ren Physiol. 2018;315:F653–64.
Mahon WA, Holland J, Urowitz MB. Hyperosmolar, non-ketotic diabetic coma. Can Med Assoc J. 1968;99:1090–2.
Hayama N, Wang W, Schneider EG. Osmolality-induced changes in aldosterone secretion involve a chloride-dependent process. Am J Physiol. 1995;268:R8–13.
Asakura-Kinoshita M, Masuda T, Oka K, Ohara K, Miura M, Morinari M, et al. Sodium-glucose cotransporter 2 inhibitor combined with conventional diuretics ameliorate body fluid retention without excessive plasma volume reduction. Diagnostics. 2024;14:1194.
Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87:1409–39.
Perkovic V, Tuttle KR, Rossing P, Mahaffey KW, Mann JFE, Bakris G, et al. Effects of semaglutide on chronic kidney disease in patients with type 2 diabetes. N Engl J Med. 2024;391:109–21.
Pratley RE, Tuttle KR, Rossing P, Rasmussen S, Perkovic V, Nielsen OW, et al. Effects of semaglutide on heart failure outcomes in diabetes and chronic kidney disease in the FLOW trial. J Am Coll Cardiol. 2024;S0735-1097:08116–6.
Vallon V, Docherty NG. Intestinal regulation of urinary sodium excretion and the pathophysiology of diabetic kidney disease: a focus on glucagon-like peptide 1 and dipeptidyl peptidase 4. Exp Physiol. 2014;99:1140–5.
Alicic RZ, Cox EJ, Neumiller JJ, Tuttle KR. Incretin drugs in diabetic kidney disease: biological mechanisms and clinical evidence. Nat Rev Nephrol. 2021;17:227–44.
Kodera R, Shikata K, Kataoka HU, Takatsuka T, Miyamoto S, Sasaki M, et al. Glucagon-like peptide-1 receptor agonist ameliorates renal injury through its anti-inflammatory action without lowering blood glucose level in a rat model of type 1 diabetes. Diabetologia. 2011;54:965–78.
Rieg T, Gerasimova M, Murray F, Masuda T, Tang T, Rose M, et al. Natriuretic effect by exendin-4, but not the DPP-4 inhibitor alogliptin, is mediated via the GLP-1 receptor and preserved in obese type 2 diabetic mice. Am J Physiol Ren Physiol. 2012;303:F963–71.
Kintscher U, Edelmann F. The non-steroidal mineralocorticoid receptor antagonist finerenone and heart failure with preserved ejection fraction. Cardiovasc Diabetol. 2023;22:162.
Shibata S, Ishizawa K, Uchida S. Mineralocorticoid receptor as a therapeutic target in chronic kidney disease and hypertension. Hypertens Res. 2017;40:221–5.
Masuda T, Nagata D. Recent advances in the management of secondary hypertension: chronic kidney disease. Hypertens Res. 2020;43:869–75.
Bakris GL, Agarwal R, Anker SD, Pitt B, Ruilope LM, Rossing P, et al. Effect of finerenone on chronic kidney disease outcomes in type 2 diabetes. N Engl J Med. 2020;383:2219–29.
Pitt B, Filippatos G, Agarwal R, Anker SD, Bakris GL, Rossing P, et al. Cardiovascular events with finerenone in kidney disease and type 2 diabetes. N Engl J Med. 2021;385:2252–63.
Solomon SD, McMurray JJV, Vaduganathan M, Claggett B, Jhund PS, Desai AS, et al. Finerenone in heart failure with mildly reduced or preserved ejection fraction. N Engl J Med. 2024. https://doi.org/10.1056/NEJMoa2407107. Online ahead of print.
Barrera-Chimal J, Estrela GR, Lechner SM, Giraud S, El Moghrabi S, Kaaki S, et al. The myeloid mineralocorticoid receptor controls inflammatory and fibrotic responses after renal injury via macrophage interleukin-4 receptor signaling. Kidney Int. 2018;93:1344–55.
Agarwal R, Kolkhof P, Bakris G, Bauersachs J, Haller H, Wada T, et al. Steroidal and non-steroidal mineralocorticoid receptor antagonists in cardiorenal medicine. Eur Heart J. 2021;42:152–61.
Bureau C, Jamme M, Schurder J, Bobot M, Robert T, Couturier A, et al. Nephrosclerosis in young patients with malignant hypertension. Nephrol Dial Transplant. 2023;38:1848–56.
Basile DP, Anderson MD, Sutton TA. Pathophysiology of acute kidney injury. Compr Physiol. 2012;2:1303–53.
Ohno Y, Kanno Y, Takenaka T. Central blood pressure and chronic kidney disease. World J Nephrol. 2016;5:90–100.
Cherney D, Lund SS, Perkins BA, Groop PH, Cooper ME, Kaspers S, et al. The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59:1860–70.
Cherney DZ, Perkins BA, Soleymanlou N, Maione M, Lai V, Lee A, et al. Renal hemodynamic effect of sodium-glucose cotransporter 2 inhibition in patients with type 1 diabetes mellitus. Circulation. 2014;129:587–97.
Thomson SC, Rieg T, Miracle C, Mansoury H, Whaley J, Vallon V, et al. Acute and chronic effects of SGLT2 blockade on glomerular and tubular function in the early diabetic rat. Am J Physiol Regul Integr Comp Physiol. 2012;302:R75–83.
Kidney Disease: Improving Global Outcomes Glomerular Diseases Work G. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int. 2021;100:S1–276.
Umemura S, Arima H, Arima S, Asayama K, Dohi Y, Hirooka Y, et al. The Japanese Society of Hypertension guidelines for the management of hypertension (JSH 2019). Hypertens Res. 2019;42:1235–481.
Japanese Society of N. Essential points from evidence-based clinical practice guideline for chronic kidney disease 2023. Clin Exp Nephrol. 2024;28:473–95.
Tada K, Nakano Y, Takahashi K, Hiyamuta H, Watanabe M, Ito K, et al. Current use of angiotensin II receptor blockers and angiotensin-converting enzyme inhibitors for hypertension in patients with chronic kidney disease with proteinuria: a cross-sectional study based on real-world data. Hypertens Res. 2024. https://doi.org/10.1038/s41440-024-01896-0. Online ahead of print.
Acknowledgements
We would like to thank Editage (www.editage.jp) for English language editing.
Author information
Authors and Affiliations
Contributions
TM conceptualized this review and drafted the manuscript. TM and DN approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Masuda, T., Nagata, D. Glomerular pressure and tubular oxygen supply: a critical dual target for renal protection. Hypertens Res 47, 3330–3337 (2024). https://doi.org/10.1038/s41440-024-01944-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41440-024-01944-9