Abstract
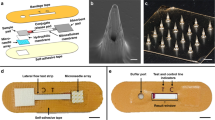
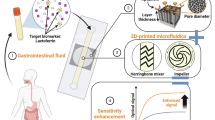
The detection and quantification of protein biomarkers in interstitial fluid is hampered by challenges in its sampling and analysis. Here we report the use of a microneedle patch for fast in vivo sampling and on-needle quantification of target protein biomarkers in interstitial fluid. We used plasmonic fluor—an ultrabright fluorescent label—to improve the limit of detection of various interstitial fluid protein biomarkers by nearly 800-fold compared with conventional fluorophores, and a magnetic backing layer to implement conventional immunoassay procedures on the patch and thus improve measurement consistency. We used the microneedle patch in mice for minimally invasive evaluation of the efficiency of a cocaine vaccine, for longitudinal monitoring of the levels of inflammatory biomarkers, and for efficient sampling of the calvarial periosteum—a challenging site for biomarker detection—and the quantification of its levels of the matricellular protein periostin, which cannot be accurately inferred from blood or other systemic biofluids. Microneedle patches for the minimally invasive collection and analysis of biomarkers in interstitial fluid might facilitate point-of-care diagnostics and longitudinal monitoring.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
118,99 € per year
only 9,92 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Data availability
The main data supporting the results in this study are available within the paper and its Supplementary Information. The raw data are available from figshare at https://doi.org/10.6084/m9.figshare.13331366.
References
Kool, J. et al. Suction blister fluid as potential body fluid for biomarker proteins. Proteomics 7, 3638–3650 (2007).
Müller, A. C. et al. A comparative proteomic study of human skin suction blister fluid from healthy individuals using immunodepletion and iTRAQ labeling. J. Proteome Res. 11, 3715–3727 (2012).
Tran, B. Q. et al. Proteomic characterization of dermal interstitial fluid extracted using a novel microneedle-assisted technique. J. Proteome Res. 17, 479–485 (2018).
Heikenfeld, J. et al. Accessing analytes in biofluids for peripheral biochemical monitoring. Nat. Biotechnol. 37, 407–419 (2019).
He, R. et al. A hydrogel microneedle patch for point-of-care testing based on skin interstitial fluid. Adv. Healthc. Mater. 9, 1901201 (2020).
Wang, Z. et al. Transdermal colorimetric patch for hyperglycemia sensing in diabetic mice. Biomaterials 237, 119782 (2020).
Gromov, P. et al. Tumor interstitial fluid—a treasure trove of cancer biomarkers. Biochim. Biophys. Acta 1834, 2259–2270 (2013).
Yang, B., Fang, X. & Kong, J. In situ sampling and monitoring cell-free DNA of the Epstein–Barr virus from dermal interstitial fluid using wearable microneedle patches. ACS Appl. Mater. Interfaces 11, 38448–38458 (2019).
Al Sulaiman, D. et al. Hydrogel-coated microneedle arrays for minimally invasive sampling and sensing of specific circulating nucleic acids from skin interstitial fluid. ACS Nano 13, 9620–9628 (2019).
McHugh, K. J. et al. Biocompatible near-infrared quantum dots delivered to the skin by microneedle patches record vaccination. Sci. Transl. Med. 11, eaay7162 (2019).
Chang, H. et al. A swellable microneedle patch to rapidly extract skin interstitial fluid for timely metabolic analysis. Adv. Mater. 29, 1702243 (2017).
Kiistala, U. Suction blister device for separation of viable epidermis from dermis. J. Invest Dermatol. 50, 129–137 (1968).
Krogstad, A., Jansson, P. A., Gisslen, P. & Lönnroth, P. Microdialysis methodology for the measurement of dermal interstitial fluid in humans. Br. J. Dermatol. 134, 1005–1012 (1996).
Bodenlenz, M. et al. Open flow microperfusion as a dermal pharmacokinetic approach to evaluate topical bioequivalence. Clin. Pharmacokinet. 56, 91–98 (2017).
Samant, P. P. & Prausnitz, M. R. Mechanisms of sampling interstitial fluid from skin using a microneedle patch. Proc. Natl Acad. Sci. USA 115, 4583–4588 (2018).
Taylor, R. M., Miller, P. R., Ebrahimi, P., Polsky, R. & Baca, J. T. Minimally-invasive, microneedle-array extraction of interstitial fluid for comprehensive biomedical applications: transcriptomics, proteomics, metabolomics, exosome research, and biomarker identification. Lab. Anim. 52, 526–530 (2018).
Muller, D. A., Corrie, S. R., Coffey, J., Young, P. R. & Kendall, M. A. Surface modified microprojection arrays for the selective extraction of the dengue virus NS1 protein as a marker for disease. Anal. Chem. 84, 3262–3268 (2012).
Coffey, J. W., Meliga, S. C., Corrie, S. R. & Kendall, M. A. Dynamic application of microprojection arrays to skin induces circulating protein extravasation for enhanced biomarker capture and detection. Biomaterials 84, 130–143 (2016).
Nedrebø, T., Reed, R. K., Jonsson, R., Berg, A. & Wiig, H. Differential cytokine response in interstitial fluid in skin and serum during experimental inflammation in rats. J. Physiol. 556, 193–202 (2004).
Zhang, X., Chen, G., Bian, F., Cai, L. & Zhao, Y. Encoded microneedle arrays for detection of skin interstitial fluid biomarkers. Adv. Mater. 31, 1902825 (2019).
Coffey, J. W., Corrie, S. R. & Kendall, M. A. Rapid and selective sampling of IgG from skin in less than 1 min using a high surface area wearable immunoassay patch. Biomaterials 170, 49–57 (2018).
Luan, J. et al. Ultrabright fluorescent nanoscale labels for the femtomolar detection of analytes with standard bioassays. Nat. Biomed. Eng. 4, 518–530 (2020).
Dulkeith, E. et al. Gold nanoparticles quench fluorescence by phase induced radiative rate suppression. Nano Lett. 5, 585–589 (2005).
Davis, S. P., Landis, B. J., Adams, Z. H., Allen, M. G. & Prausnitz, M. R. Insertion of microneedles into skin: measurement and prediction of insertion force and needle fracture force. J. Biomech. 37, 1155–1163 (2004).
Kampman, K. M. The treatment of cocaine use disorder. Sci. Adv. 5, eaax1532 (2019).
Shorter, D. & Kosten, T. R. Novel pharmacotherapeutic treatments for cocaine addiction. BMC Med. 9, 119 (2011).
Kinsey, B. M., Kosten, T. R. & Orson, F. M. Anti-cocaine vaccine development. Expert Rev. Vaccines 9, 1109–1114 (2010).
Martell, B. et al. Cocaine vaccine for the treatment of cocaine dependence: a randomized double-blind placebo-controlled efficacy trial. Arch. Gen. Psych. 66, 1116–1123 (2009).
Scott, E. A., Karabin, N. B. & Augsornworawat, P. Overcoming immune dysregulation with immunoengineered nanobiomaterials. Annu. Rev. Biomed. Eng. 19, 57–84 (2017).
Taylor, J., Laudenbach, M., Tucker, A., Jenkins, M. & Pravetoni, M. Hapten-specific naive B cells are biomarkers of vaccine efficacy against drugs of abuse. J. Immunol. Methods 405, 74–86 (2014).
Monteiro-Riviere, N. A., Bristol, D. G., Manning, T. O., Rogers, R. A. & Riviere, J. E. Interspecies and interregional analysis of the comparative histologic thickness and laser Doppler blood flow measurements at five cutaneous sites in nine species. J. Invest. Dermatol. 95, 582-6 (1990).
Davidson, A., Al-Qallaf, B. & Das, D. B. Transdermal drug delivery by coated microneedles: geometry effects on effective skin thickness and drug permeability. Chem. Eng. Res. Des. 86, 1196–1206 (2008).
Copeland, S., Warren, H. S., Lowry, S. F., Calvano, S. E. & Remick, D. Acute inflammatory response to endotoxin in mice and humans. Clin. Diagn. Lab. Immunol. 12, 60–67 (2005).
Hong, Y.-H., Chao, W.-W., Chen, M.-L. & Lin, B.-F. Ethyl acetate extracts of alfalfa (Medicago sativa L.) sprouts inhibit lipopolysaccharide-induced inflammation in vitro and in vivo. J. Biomed. Sci. 16, 64 (2009).
Colnot, C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J. Bone Miner. Res. 24, 274–282 (2009).
Dwek, J. R. The periosteum: what is it, where is it, and what mimics it in its absence? Skelet. Radiol. 39, 319–323 (2010).
Sakai, D. et al. Remodeling of actin cytoskeleton in mouse periosteal cells under mechanical loading induces periosteal cell proliferation during bone formation. PLoS ONE 6, e24847 (2011).
Moore, S. R., Milz, S. & Knothe Tate, M. L. Periosteal thickness and cellularity in mid-diaphyseal cross‐sections from human femora and tibiae of aged donors. J. Anat. 224, 142–149 (2014).
Merle, B. & Garnero, P. The multiple facets of periostin in bone metabolism. Osteoporos. Int. 23, 1199–1212 (2012).
Kyutoku, M. et al. Role of periostin in cancer progression and metastasis: inhibition of breast cancer progression and metastasis by anti-periostin antibody in a murine model. Int. J. Mol. Med. 28, 181–186 (2011).
Yan, J. et al. Circulating periostin levels increase in association with bone density loss and healing progression during the early phase of hip fracture in Chinese older women. Osteoporos. Int. 28, 2335–2341 (2017).
Bonnet, N., Garnero, P. & Ferrari, S. Special Issue on bone disease mechanisms: periostin action in bone. Mol. Cell. Endocrinol. 432, 75–82 (2015).
Cai, Y. et al. Magnet patterned superparamagnetic Fe3O4/Au core–shell nanoplasmonic sensing array for label-free high throughput cytokine immunoassay. Adv. Healthc. Mater. 8, 1801478 (2019).
Hu, M. et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem. Soc. Rev. 35, 1084–1094 (2006).
Lee, K.-S. & El-Sayed, M. A. Dependence of the enhanced optical scattering efficiency relative to that of absorption for gold metal nanorods on aspect ratio, size, end-cap shape, and medium refractive index. J. Phys. Chem. B 109, 20331–20338 (2005).
Gole, A. & Murphy, C. J. Azide-derivatized gold nanorods: functional materials for ‘click’ chemistry. Langmuir 24, 266–272 (2008).
Tebbe, M., Kuttner, C., Männel, M., Fery, A. & Chanana, M. Colloidally stable and surfactant-free protein-coated gold nanorods in biological media. ACS Appl. Mater. Interfaces 7, 5984–5991 (2015).
Acknowledgements
We acknowledge support from the National Science Foundation (CBET-1900277) and the National Institutes of Health (R01DE027098, R56DE027924, R01CA141521, R21DA036663, R21CA236652). We thank the Nano Research Facility and Institute of Materials Science and Engineering at Washington University for providing access to electron microscopy facilities, N. Huebsch for providing access to the fluorescence microscope, K. Magee for the help with mouse experiments, Y. Diao for help with digital photographs and M. Shen for inspiring discussions.
Author information
Authors and Affiliations
Contributions
S.S., Z.W. and J.L. conceived the project. S.S., J.S.R., E.L.S., Z.W. and J.L. designed the experiments. Z.W., J.L. and M.Y. fabricated the microneedle patches. Z.W., A.S., L.L., X.Z., J.S.R. and E.L.S. performed the animal experiments. Z.W., J.L., A.S., L.L., Q.Z. and J.J.M. performed the bioassays. J.L. and Q.J. synthesized the plasmonic fluors. P.G. synthesized the magnetic nanoparticles. R.G. performed TEM imaging. P.R. and Y.W. performed SEM imaging. S.C. collected fluorescence images of the microneedles. S.S., J.S.R., E.L.S., J.J.M., Z.W. and J.L. wrote the paper. All authors reviewed and commented on the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare the following competing financial interests: J.L., J.J.M. and S.S. are inventors on a provisional patent related to plasmonic fluor technology and the technology has been licensed by the Office of Technology Management at Washington University in St Louis to Auragent Bioscience LLC, which is developing plasmonic fluor products. J.L., J.J.M. and S.S. are co-founders and shareholders of Auragent Bioscience LLC. These potential conflicts of interest have been disclosed and are being managed by Washington University in St Louis.
Additional information
Peer review information Peer reviewer reports are available.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Wang, Z., Luan, J., Seth, A. et al. Microneedle patch for the ultrasensitive quantification of protein biomarkers in interstitial fluid. Nat Biomed Eng 5, 64–76 (2021). https://doi.org/10.1038/s41551-020-00672-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41551-020-00672-y
This article is cited by
-
Imaging Brain Interstitial Fluid
Sensing and Imaging (2025)
-
Advancements in transdermal drug delivery using microneedles: technological and material perspective
Discover Pharmaceutical Sciences (2025)
-
Polymeric Microneedles for Biomedical Applications: Innovations in Transdermal Drug Delivery and Biosensing Technologies
Biomedical Materials & Devices (2025)
-
Recent advances and perspectives of MicroNeedles for biomedical applications
Biophysical Reviews (2025)
-
Microneedle electrodes: materials, fabrication methods, and electrophysiological signal monitoring-narrative review
Biomedical Microdevices (2025)