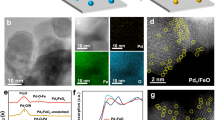
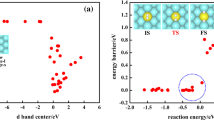
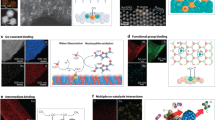
Abstract
Single-atom catalysts (SACs) have attracted considerable research interest owing to their combined merits of homogeneous and heterogeneous catalysts. However, the uniform and isolated active sites of SACs fall short in catalysing complex chemical processes that simultaneously involve multiple intermediates. In this Review, we highlight an emerging class of catalysts with adjacent binary active centres, which is called integrative catalytic pairs (ICPs), showing not only atomic-scale site-to-site electronic interactions but also synergistic catalytic effects. Compared with SACs or their derivative dual-atom catalysts (DACs), multi-interactive intermediates on ICPs can overcome kinetic barriers, adjust reaction pathways and break the universal linear scaling relations as the smallest active units. Starting from this active-site design principle, each single active atom can be considered as a brick to further build integrative catalytic clusters (ICCs) with desirable configurations, towards trimer or even larger multi-atom units depending on the requirement of a given reaction.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
269,00 € per year
only 22,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vogt, C. & Weckhuysen, B. M. The concept of active site in heterogeneous catalysis. Nat. Rev. Chem. 6, 89–111 (2022).
Qiao, B. et al. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 3, 634–641 (2011). This report introduced the concept of SACs, in which single Pt atoms on a FeOx support showed high activity and stability for CO oxidation.
Yang, X.-F. et al. Single-atom catalysts: a new frontier in heterogeneous catalysis. Acc. Chem. Res. 46, 1740–1748 (2013).
Wang, A., Li, J. & Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2, 65–81 (2018).
Liu, L. & Corma, A. Metal catalysts for heterogeneous catalysis: from single atoms to nanoclusters and nanoparticles. Chem. Rev. 118, 4981–5079 (2018).
Kyriakou, G. et al. Isolated metal atom geometries as a strategy for selective heterogeneous hydrogenations. Science 335, 1209–1212 (2012).
Yang, J., Li, W., Wang, D. & Li, Y. Electronic metal–support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 32, 2003300 (2020).
Nie, L. et al. Activation of surface lattice oxygen in single-atom Pt/CeO2 for low-temperature CO oxidation. Science 358, 1419–1423 (2017).
Lin, L. et al. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts. Nature 544, 80–83 (2017).
Liu, D. et al. Atomically dispersed platinum supported on curved carbon supports for efficient electrocatalytic hydrogen evolution. Nat. Energy 4, 512–518 (2019).
Xiong, Y. et al. Single-atom Rh/N-doped carbon electrocatalyst for formic acid oxidation. Nat. Nanotechnol. 15, 390–397 (2020).
Mehmood, A. et al. High loading of single atomic iron sites in Fe–NC oxygen reduction catalysts for proton exchange membrane fuel cells. Nat. Catal. 5, 311–323 (2022).
Teng, Z. et al. Atomically dispersed antimony on carbon nitride for the artificial photosynthesis of hydrogen peroxide. Nat. Catal. 4, 374–384 (2021).
Tan, H. et al. Photocatalysis of water into hydrogen peroxide over an atomic Ga–N5 site. Nat. Synth. 2, 557–563 (2023).
Ji, S. et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 4, 407–417 (2021).
Liu, Y. et al. Recent advances in carbon-supported noble-metal electrocatalysts for hydrogen evolution reaction: syntheses, structures, and properties. Adv. Energy Mater. 12, 2200928 (2022).
Wei, H. et al. FeOx-supported platinum single-atom and pseudo-single-atom catalysts for chemoselective hydrogenation of functionalized nitroarenes. Nat. Commun. 5, 5634 (2014).
Sun, K., Shan, H., Neumann, H., Lu, G.-P. & Beller, M. Efficient iron single-atom catalysts for selective ammoxidation of alcohols to nitriles. Nat. Commun. 13, 1848 (2022).
Zhao, J. et al. A heterogeneous iridium single-atom-site catalyst for highly regioselective carbenoid O–H bond insertion. Nat. Catal. 4, 523–531 (2021).
Chen, Z. et al. A heterogeneous single-atom palladium catalyst surpassing homogeneous systems for Suzuki coupling. Nat. Nanotechnol. 13, 702–707 (2018).
Bajada, M. A. et al. Light-driven C–O coupling of carboxylic acids and alkyl halides over a Ni single-atom catalyst. Nat. Synth. 2, 1092–1103 (2023).
Yang, H. B. et al. Atomically dispersed Ni(I) as the active site for electrochemical CO2 reduction. Nat. Energy 3, 140–147 (2018).
Jung, E. et al. Atomic-level tuning of Co–N–C catalyst for high-performance electrochemical H2O2 production. Nat. Mater. 19, 436–442 (2020).
Zheng, T. et al. Copper-catalysed exclusive CO2 to pure formic acid conversion via single-atom alloying. Nat. Nanotechnol. 16, 1386–1393 (2021). This work investigated a single-atom Pb-alloyed Cu catalyst in the electrochemical CO2RR and revealed that isolated Pb atoms precisely tune the electronic/geometric structure of the Cu catalyst but can not work as active sites.
Datye, A. K. & Guo, H. Single atom catalysis poised to transition from an academic curiosity to an industrially relevant technology. Nat. Commun. 12, 895 (2021).
Li, X., Yang, X., Zhang, J., Huang, Y. & Liu, B. In situ/operando techniques for characterization of single-atom catalysts. ACS Catal. 9, 2521–2531 (2019).
Hulva, J. et al. Unraveling CO adsorption on model single-atom catalysts. Science 371, 375–379 (2021).
Cao, H. et al. Engineering single-atom electrocatalysts for enhancing kinetics of acidic volmer reaction. J. Am. Chem. Soc. 145, 13038–13047 (2023).
Yang, H. B. et al. Identification of non-metal single atomic phosphorus active sites for the CO2 reduction reaction. EES Catal. 1, 774–783 (2023). This work extended the definition of SACs to a family of non-metal catalytic centres.
Gu, Y., Xi, B. J., Zhang, H., Ma, Y. C. & Xiong, S. L. Activation of main-group antimony atomic sites for oxygen reduction catalysis. Angew. Chem. Int. Ed. 61, e202202200 (2022).
Zhao, Y. et al. Non-metal single-iodine-atom electrocatalysts for the hydrogen evolution reaction. Angew. Chem. Int. Ed. 58, 12252–12257 (2019).
Fu, W. et al. Non-metal single-phosphorus-atom catalysis of hydrogen evolution. Angew. Chem. Int. Ed. 59, 23791–23799 (2020).
Ding, K. et al. Identification of active sites in CO oxidation and water-gas shift over supported Pt catalysts. Science 350, 189–192 (2015).
Li, M. et al. Single-atom tailoring of platinum nanocatalysts for high-performance multifunctional electrocatalysis. Nat. Catal. 2, 495–503 (2019).
Wang, Q. et al. Coordination engineering of iridium nanocluster bifunctional electrocatalyst for highly efficient and pH-universal overall water splitting. Nat. Commun. 11, 4246 (2020).
Wang, P. et al. Breaking scaling relations to achieve low-temperature ammonia synthesis through LiH-mediated nitrogen transfer and hydrogenation. Nat. Chem. 9, 64–70 (2017).
Campos, J. Bimetallic cooperation across the periodic table. Nat. Rev. Chem. 4, 696–702 (2020).
Zhang, W. et al. Emerging dual-atomic-site catalysts for efficient energy catalysis. Adv. Mater. 33, 2102576 (2021).
Li, W.-H., Yang, J. & Wang, D. Long-range interactions in diatomic catalysts boosting electrocatalysis. Angew. Chem. Int. Ed. 61, e202213318 (2022).
Zhu, P., Xiong, X., Wang, D. & Li, Y. Advances and regulation strategies of the active moiety in dual-atom site catalysts for efficient electrocatalysis. Adv. Energy Mater. 13, 2300884 (2023).
Wang, Q. et al. Atomic metal–non-metal catalytic pair drives efficient hydrogen oxidation catalysis in fuel cells. Nat. Catal. 6, 916–926 (2023). This study represented the first definition and application of ICPs, in which the reactive *OH species adsorbed on the more oxophilic P site induced an alternative thermodynamic pathway to facilely combine with the *H on the adjacent Ir atom, thus synergistically boosting the performance for HOR in fuel cells.
He, Q. et al. Electrochemical conversion of CO2 to syngas with controllable CO/H2 ratios over Co and Ni single-atom catalysts. Angew. Chem. Int. Ed. 59, 3033–3037 (2020).
Zhao, Y. et al. Simultaneous oxidative and reductive reactions in one system by atomic design. Nat. Catal. 4, 134–143 (2021). By integrating two compatible single-atom systems of Pd and Fe as a yolk–shell structure, this catalyst simultaneously catalysed nitroaromatic hydrogenation and alkene epoxidation reactions, leading to a cascade synthesis of amino alcohols.
Chen, J. et al. Dual single-atomic Ni–N4 and Fe–N4 sites constructing Janus hollow graphene for selective oxygen electrocatalysis. Adv. Mater. 32, 2003134 (2020).
Tang, C. et al. Tailoring acidic oxygen reduction selectivity on single-atom catalysts via modification of first and second coordination spheres. J. Am. Chem. Soc. 143, 7819–7827 (2021).
Chang, X. et al. Designing single-site alloy catalysts using a degree-of-isolation descriptor. Nat. Nanotechnol. 18, 611–616 (2023).
Jin, Z. et al. Understanding the inter-site distance effect in single-atom catalysts for oxygen electroreduction. Nat. Catal. 4, 615–622 (2021).
Jiao, L. et al. Non-bonding interaction of neighboring Fe and Ni single-atom pairs on MOF-derived N-doped carbon for enhanced CO2 electroreduction. J. Am. Chem. Soc. 143, 19417–19424 (2021).
Luo, F. et al. Structural and reactivity effects of secondary metal doping into iron-nitrogen-carbon catalysts for oxygen electroreduction. J. Am. Chem. Soc. 145, 14737–14747 (2023).
Zhang, L. et al. Atomic layer deposited Pt-Ru dual-metal dimers and identifying their active sites for hydrogen evolution reaction. Nat. Commun. 10, 4936 (2019).
Cui, T. et al. Engineering dual single-atom sites on 2D ultrathin N-doped carbon nanosheets attaining ultra-low-temperature zinc–air battery. Angew. Chem. Int. Ed. 61, e202115219 (2022).
Yan, H. et al. Bottom-up precise synthesis of stable platinum dimers on graphene. Nat. Commun. 8, 1070 (2017).
Jiang, Z. et al. Interlayer-confined NiFe dual atoms within MoS2 electrocatalyst for ultra-efficient acidic overall water splitting. Adv. Mater. 35, 2300505 (2023).
Li, Y. et al. Atomically dispersed dual-metal site catalysts for enhanced CO2 reduction: mechanistic insight into active site structures. Angew. Chem. Int. Ed. 61, e202205632 (2022).
Zhang, N. et al. High-density planar-like Fe2N6 structure catalyzes efficient oxygen reduction. Matter 3, 509–521 (2020).
Jiao, J. et al. Copper atom-pair catalyst anchored on alloy nanowires for selective and efficient electrochemical reduction of CO2. Nat. Chem. 11, 222–228 (2019). This work reported a homologous binuclear DAC featuring stable Cu10−Cu1x+ pair configurations, with Cu10 adsorbing CO2 and the neighbouring Cu1x+ adsorbing H2O, which thereby worked together to promote the critical bimolecular step in CO2 reduction.
Hao, Q. et al. Nickel dual-atom sites for electrochemical carbon dioxide reduction. Nat. Synth. 1, 719–728 (2022).
Hai, X. et al. Geminal-atom catalysis for cross-coupling. Nature 622, 754–760 (2023).
Li, H. et al. Synergetic interaction between neighbouring platinum monomers in CO2 hydrogenation. Nat. Nanotechnol. 13, 411–417 (2018). This study discovered the synergetic interaction between neighbouring Pt monomers that reduced the activation energy barrier and underwent distinct reaction paths relative to isolated monomers.
Wang, J. et al. Design of N-coordinated dual-metal sites: a stable and active Pt-free catalyst for acidic oxygen reduction reaction. J. Am. Chem. Soc. 139, 17281–17284 (2017).
Zhang, X. et al. Identifying and tailoring C–N coupling site for efficient urea synthesis over diatomic Fe–Ni catalyst. Nat. Commun. 13, 5337 (2022).
Li, X. et al. Palladium and ruthenium dual-single-atom sites on porous ionic polymers for acetylene dialkoxycarbonylation: synergetic effects stabilize the active site and increase CO adsorption. Angew. Chem. Int. Ed. 62, e202307570 (2023).
Fang, C. et al. Synergy of dual-atom catalysts deviated from the scaling relationship for oxygen evolution reaction. Nat. Commun. 14, 4449 (2023).
Zhao, Q.-P. et al. Photo-induced synthesis of heteronuclear dual-atom catalysts. Nat. Synth. 3, 497–506 (2024). This work proposed a universal ‘navigation and positioning’ method to precisely and scalably synthesize a series of heteronuclear DACs and demonstrated outstanding photocatalytic HER activity for as-prepared Zn1–Ru1/DACs.
Du, J. et al. CoIn dual-atom catalyst for hydrogen peroxide production via oxygen reduction reaction in acid. Nat. Commun. 14, 4766 (2023).
Zhang, S. et al. Atomically dispersed bimetallic Fe–Co electrocatalysts for green production of ammonia. Nat. Sustain. 6, 169–179 (2023).
Young, D. Computational Chemistry: A Practical Guide for Applying Techniques to Real World Problems (Wiley, 2001).
Ding, J. et al. Room-temperature chemoselective hydrogenation of nitroarene over atomic metal–nonmetal catalytic pair. Adv. Mater. 36, 2306480 (2024).
Zhang, Q. et al. Boosting the proton-coupled electron transfer via Fe−P atomic pair for enhanced electrochemical CO2 reduction. Angew. Chem. Int. Ed. 62, e202311550 (2023).
Ding, J. et al. A tin-based tandem electrocatalyst for CO2 reduction to ethanol with 80% selectivity. Nat. Energy 8, 1386–1394 (2023). This study reported that an ICP comprising Sn and O active sites could adsorb *CHO and *CO(OH) intermediates, respectively, therefore promoting C−C bond formation through a tandem formyl-bicarbonate coupling pathway in electrochemical CO2 reduction to ethanol.
Ding, J. et al. Circumventing CO2 reduction scaling relations over the heteronuclear diatomic catalytic pair. J. Am. Chem. Soc. 145, 11829–11836 (2023). In this report, the adsorption configuration transitioned from the bridge configuration of CO2 on Fe1–Mo1/ICP to the linear configuration of CO on the Fe1 centre, which resulted in breaking the scaling relationship in the CO2RR, simultaneously promoting CO2 activation and CO release.
Bligaard, T. et al. The Brønsted–Evans–Polanyi relation and the volcano curve in heterogeneous catalysis. J. Catal. 224, 206–217 (2004).
Abild-Pedersen, F. et al. Scaling properties of adsorption energies for hydrogen-containing molecules on transition-metal surfaces. Phys. Rev. Lett. 99, 016105 (2007).
Koper, M. T. M. Thermodynamic theory of multi-electron transfer reactions: Implications for electrocatalysis. J. Electroanal. Chem. 660, 254–260 (2011).
Gao, R. et al. Pt/Fe2O3 with Pt–Fe pair sites as a catalyst for oxygen reduction with ultralow Pt loading. Nat. Energy 6, 614–623 (2021).
Ro, I. et al. Bifunctional hydroformylation on heterogeneous Rh–WOx pair site catalysts. Nature 609, 287–292 (2022).
Zeng, L. et al. Cooperative Rh–O5/Ni(Fe) site for efficient biomass upgrading coupled with H2 production. J. Am. Chem. Soc. 145, 17577–17587 (2023).
Zhou, Y. et al. Peripheral-nitrogen effects on the Ru1 centre for highly efficient propane dehydrogenation. Nat. Catal. 5, 1145–1156 (2022).
Xia, W. et al. Adjacent copper single atoms promote C–C coupling in electrochemical CO2 reduction for the efficient conversion of ethanol. J. Am. Chem. Soc. 145, 17253–17264 (2023).
Yang, Y. et al. O-coordinated W–Mo dual-atom catalyst for pH-universal electrocatalytic hydrogen evolution. Sci. Adv. 6, eaba6586 (2020).
Bai, L., Hsu, C.-S., Alexander, D. T. L., Chen, H. M. & Hu, X. Double-atom catalysts as a molecular platform for heterogeneous oxygen evolution electrocatalysis. Nat. Energy 6, 1054–1066 (2021).
Chen, Y. et al. Isolating single and few atoms for enhanced catalysis. Adv. Mater. 34, 2201796 (2022).
Wang, L. et al. A sulfur-tethering synthesis strategy toward high-loading atomically dispersed noble metal catalysts. Sci. Adv. 5, eaax6322 (2019).
Wang, Q. et al. Long-term stability challenges and opportunities in acidic oxygen evolution electrocatalysis. Angew. Chem. Int. Ed. 62, e202216645 (2023).
Liu, L. & Corma, A. Identification of the active sites in supported subnanometric metal catalysts. Nat. Catal. 4, 453–456 (2021).
Ajayi, T. M. et al. Characterization of just one atom using synchrotron X-rays. Nature 618, 69–73 (2023).
Green, I. X., Tang, W., Neurock, M. & Yates, J. T. Spectroscopic observation of dual catalytic sites during oxidation of CO on a Au/TiO2 catalyst. Science 333, 736–739 (2011).
Wei, S. et al. Direct observation of noble metal nanoparticles transforming to thermally stable single atoms. Nat. Nanotechnol. 13, 856–861 (2018).
Hartman, T., Geitenbeek, R. G., Whiting, G. T. & Weckhuysen, B. M. Operando monitoring of temperature and active species at the single catalyst particle level. Nat. Catal. 2, 986–996 (2019).
Maurer, F. et al. Tracking the formation, fate and consequence for catalytic activity of Pt single sites on CeO2. Nat. Catal. 3, 824–833 (2020).
Zhong, M. et al. Accelerated discovery of CO2 electrocatalysts using active machine learning. Nature 581, 178–183 (2020).
Liu, J.-C. et al. Heterogeneous Fe3 single-cluster catalyst for ammonia synthesis via an associative mechanism. Nat. Commun. 9, 1610 (2018).
Wang, G., Jiang, X.-L., Jiang, Y.-F., Wang, Y.-G. & Li, J. Screened Fe3 and Ru3 single-cluster catalysts anchored on MoS2 supports for selective hydrogenation of CO2. ACS Catal. 13, 8413–8422 (2023).
Han, L. et al. A single-atom library for guided monometallic and concentration-complex multimetallic designs. Nat. Mater. 21, 681–688 (2022).
Acknowledgements
This work was financially supported by the City University of Hong Kong startup fund (no. 9020003) and ITF−RTH−Global STEM Professorship (no. 9446006). H.B.Y. acknowledges support from the National Natural Science Foundation of China under grant number 22075195. C.S. gratefully thanks financial support from National Key Research and Development Program of China (number 2021YFA1600800633), National Natural Science Foundation of China (number 22372102) and Shenzhen Science and Technology Program (number RCJC20200714114434086).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Nanotechnology thanks Shuangyin Wang and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, Q., Cheng, Y., Yang, H.B. et al. Integrative catalytic pairs for efficient multi-intermediate catalysis. Nat. Nanotechnol. 19, 1442–1451 (2024). https://doi.org/10.1038/s41565-024-01716-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41565-024-01716-z
This article is cited by
-
Breaking the linear scaling limit in multi-electron-transfer electrocatalysis through intermediate spillover
Nature Catalysis (2025)
-
The geometric-electronic coupled design of diatomic catalyst towards oxygen reduction reaction
Nature Communications (2025)
-
Electrically reconfigurable heteronuclear dual-atom catalysts
Nature Nanotechnology (2025)
-
Atomic-scale Observation of the Generation and Dispersion of Iron Single Atoms
Chemical Research in Chinese Universities (2025)
-
Single metal, dual sites: Co-P moieties enable efficient and stable electrochemical hydrogen production
Science China Chemistry (2025)