Abstract
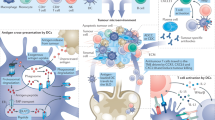
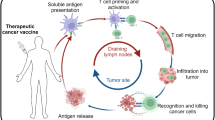
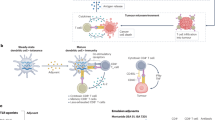
In situ cancer vaccination refers to any approach that exploits tumour antigens available at a tumour site to induce tumour-specific adaptive immune responses. These approaches hold great promise for the treatment of many solid tumours, with numerous candidate drugs under preclinical or clinical evaluation and several products already approved. However, there are challenges in the development of effective in situ cancer vaccines. For example, inadequate release of tumour antigens from tumour cells limits antigen uptake by immune cells; insufficient antigen processing by antigen-presenting cells restricts the generation of antigen-specific T cell responses; and the suppressive immune microenvironment of the tumour leads to exhaustion and death of effector cells. Rationally designed delivery technologies such as lipid nanoparticles, hydrogels, scaffolds and polymeric nanoparticles are uniquely suited to overcome these challenges through the targeted delivery of therapeutics to tumour cells, immune cells or the extracellular matrix. Here, we discuss delivery technologies that have the potential to reduce various clinical barriers for in situ cancer vaccines. We also provide our perspective on this emerging field that lies at the interface of cancer vaccine biology and delivery technologies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Saxena, M., van der Burg, S. H., Melief, C. J. & Bhardwaj, N. Therapeutic cancer vaccines. Nat. Rev. Cancer 21, 360–378 (2021).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer–immunity cycle. Immunity 39, 1–10 (2013).
Li, Q. et al. Symphony of nanomaterials and immunotherapy based on the cancer–immunity cycle. Acta Pharm. Sin. B 12, 107–134 (2022).
Duan, X., Chan, C. & Lin, W. Nanoparticle-mediated immunogenic cell death enables and potentiates cancer immunotherapy. Angew. Chem. Int. Ed. 58, 670–680 (2019).
Hammerich, L., Binder, A. & Brody, J. D. In situ vaccination: cancer immunotherapy both personalized and off-the-shelf. Mol. Oncol. 9, 1966–1981 (2015).
Formenti, S. C. & Demaria, S. Radiation therapy to convert the tumor into an in situ vaccine. Int. J. Radiat. Oncol. Biol. Phys. 84, 879–880 (2012).
Grass, G. D., Krishna, N. & Kim, S. The immune mechanisms of abscopal effect in radiation therapy. Curr. Probl. Cancer 40, 10–24 (2016).
Sheen, M. R. & Fiering, S. In situ vaccination: harvesting low hanging fruit on the cancer immunotherapy tree. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11, e1524 (2019).
Lamm, D. L. et al. Incidence and treatment of complications of bacillus Calmette–Guerin intravesical therapy in superficial bladder cancer. J. Urol. 147, 596–600 (1992).
Li, J., Zhan, L. & Qin, C. The double-sided effects of Mycobacterium bovis bacillus Calmette–Guérin vaccine. NPJ Vaccines 6, 14 (2021).
Adams, S. et al. Topical TLR7 agonist imiquimod can induce immune-mediated rejection of skin metastases in patients with breast cancer. Clin. Cancer Res. 18, 6748–6757 (2012).
Vacchelli, E. et al. Trial watch: FDA-approved Toll-like receptor agonists for cancer therapy. Oncoimmunology 1, 894–907 (2012).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Yuan, B., Wang, G., Tang, X., Tong, A. & Zhou, L. Immunotherapy of glioblastoma: recent advances and future prospects. Hum. Vaccin. Immunother. 18, 2055417 (2022).
Svensson-Arvelund, J. et al. Expanding cross-presenting dendritic cells enhances oncolytic virotherapy and is critical for long-term anti-tumor immunity. Nat. Commun. 13, 7149 (2022).
Gerken, L. R., Gerdes, M. E., Pruschy, M. & Herrmann, I. K. Prospects of nanoparticle-based radioenhancement for radiotherapy. Mater. Horiz. 10, 4059–4082 (2023).
Hammerich, L. et al. Systemic clinical tumor regressions and potentiation of PD1 blockade with in situ vaccination. Nat. Med. 25, 814–824 (2019).
Hong, W. X. et al. Intratumoral immunotherapy for early-stage solid tumors. Clin. Cancer Res. 26, 3091–3099 (2020).
Blankenstein, T., Coulie, P. G., Gilboa, E. & Jaffee, E. M. The determinants of tumour immunogenicity. Nat. Rev. Cancer 12, 307–313 (2012).
Jhunjhunwala, S., Hammer, C. & Delamarre, L. Antigen presentation in cancer: insights into tumour immunogenicity and immune evasion. Nat. Rev. Cancer 21, 298–312 (2021).
Munn, D. H. & Bronte, V. Immune suppressive mechanisms in the tumor microenvironment. Curr. Opin. Immunol. 39, 1–6 (2016).
Riley, R. S., June, C. H., Langer, R. & Mitchell, M. J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 18, 175–196 (2019).
Patra, J. K. et al. Nano based drug delivery systems: recent developments and future prospects. J. Nanobiotechnology 16, 71 (2018).
Bozzuto, G. & Molinari, A. Liposomes as nanomedical devices. Int. J. Nanomed. 10, 975 (2015).
Begines, B. et al. Polymeric nanoparticles for drug delivery: recent developments and future prospects. Nanomaterials 10, 1403 (2020).
Hou, X., Zaks, T., Langer, R. & Dong, Y. Lipid nanoparticles for mRNA delivery. Nat. Rev. Mater. 6, 1078–1094 (2021).
Gaumet, M., Vargas, A., Gurny, R. & Delie, F. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 69, 1–9 (2008).
Truong, N. P., Whittaker, M. R., Mak, C. W. & Davis, T. P. The importance of nanoparticle shape in cancer drug delivery. Expert Opin. Drug Deliv. 12, 129–142 (2015).
Ha, C.-S. & Gardella, J. A. Surface chemistry of biodegradable polymers for drug delivery systems. Chem. Rev. 105, 4205–4232 (2005).
Liu, Q., Guan, J., Qin, L., Zhang, X. & Mao, S. Physicochemical properties affecting the fate of nanoparticles in pulmonary drug delivery. Drug Discov. Today 25, 150–159 (2020).
Cho, K., Wang, X., Nie, S., Chen, Z. & Shin, D. M. Therapeutic nanoparticles for drug delivery in cancer. Clin. Cancer Res. 14, 1310–1316 (2008).
Malaviya, P., Shukal, D. & Vasavada, A. R. Nanotechnology-based drug delivery, metabolism and toxicity. Curr. Drug Metab. 20, 1167–1190 (2019).
Maeda, H. Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects. Bioconjug. Chem. 21, 797–802 (2010).
Cheng, Q. et al. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 15, 313–320 (2020).
Shafei, A. et al. A review on the efficacy and toxicity of different doxorubicin nanoparticles for targeted therapy in metastatic breast cancer. Biomed. Pharmacother. 95, 1209–1218 (2017).
El-Sawy, H. S., Al-Abd, A. M., Ahmed, T. A., El-Say, K. M. & Torchilin, V. P. Stimuli-responsive nano-architecture drug-delivery systems to solid tumor micromilieu: past, present, and future perspectives. ACS Nano 12, 10636–10664 (2018).
Qiao, Y. et al. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 11, e1527 (2019).
Green, D. R., Ferguson, T., Zitvogel, L. & Kroemer, G. Immunogenic and tolerogenic cell death. Nat. Rev. Immunol. 9, 353–363 (2009).
Ouyang, L. et al. Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif. 45, 487–498 (2012).
Shewach, D. S. & Kuchta, R. D. Introduction to cancer chemotherapeutics. Chem. Rev. 109, 2859–2861 (2009).
Liu, T., Yang, K. & Liu, Z. Recent advances in functional nanomaterials for X-ray triggered cancer therapy. Prog. Nat. Sci. Mater. 30, 567–576 (2020).
Yakkala, C., Denys, A., Kandalaft, L. & Duran, R. Cryoablation and immunotherapy of cancer. Curr. Opin. Biotechnol. 65, 60–64 (2020).
Zhou, Q. et al. Mannose-derived carbon dots amplify microwave ablation-induced antitumor immune responses by capturing and transferring “danger signals” to dendritic cells. ACS Nano 15, 2920–2932 (2021).
Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol. 17, 657–674 (2020).
Antonio Chiocca, E. Oncolytic viruses. Nat. Rev. Cancer 2, 938–950 (2002).
Pan, H., Soman, N. R., Schlesinger, P. H., Lanza, G. M. & Wickline, S. A. Cytolytic peptide nanoparticles (‘NanoBees’) for cancer therapy. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 3, 318–327 (2011).
Laviano, A. & Rossi Fanelli, F. Toxicity in chemotherapy — when less is more. N. Engl. J. Med. 366, 2319–2320 (2012).
Yan, L. et al. Nanoparticle-based drug delivery system: a patient-friendly chemotherapy for oncology. Dose Response 18, 1559325820936161 (2020).
Rezaei-Tavirani, M. et al. TiO2 nanoparticle as a sensitizer drug in radiotherapy: in vitro study. Int. J. Cancer 6, e80460 (2013).
Fu, L.-Q. et al. Recent advances in oncolytic virus-based cancer therapy. Virus Res. 270, 197675 (2019).
Kennedy, E. M. et al. Development of intravenously administered synthetic RNA virus immunotherapy for the treatment of cancer. Nat. Commun. 13, 5907 (2022). LNPs encapsulating an mRNA encoding oncolytic virus can shield the virus from immune clearance and improve in situ vaccination.
DeVita, V. T. Jr & Chu, E. A history of cancer chemotherapy. Cancer Res. 68, 8643–8653 (2008).
D’arcy, M. S. Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 43, 582–592 (2019).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Vanmeerbeek, I. et al. Trial watch: chemotherapy-induced immunogenic cell death in immuno-oncology. Oncoimmunology 9, 1703449 (2020).
Blanco, E., Shen, H. & Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 33, 941–951 (2015).
Banfi, A. et al. High-dose chemotherapy shows a dose-dependent toxicity to bone marrow osteoprogenitors: a mechanism for post–bone marrow transplantation osteopenia. Cancer 92, 2419–2428 (2001).
Shi, Y., Van der Meel, R., Chen, X. & Lammers, T. The EPR effect and beyond: strategies to improve tumor targeting and cancer nanomedicine treatment efficacy. Theranostics 10, 7921 (2020).
Fang, J., Nakamura, H. & Maeda, H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv. Drug Deliv. Rev. 63, 136–151 (2011).
Liu, X. et al. Mixed‐charge nanoparticles for long circulation, low reticuloendothelial system clearance, and high tumor accumulation. Adv. Healthc. Mater. 3, 1439–1447 (2014).
Jokerst, J. V., Lobovkina, T., Zare, R. N. & Gambhir, S. S. Nanoparticle PEGylation for imaging and therapy. Nanomedicine 6, 715–728 (2011).
Chidambaram, M., Manavalan, R. & Kathiresan, K. Nanotherapeutics to overcome conventional cancer chemotherapy limitations. J. Pharm. Pharm. Sci. 14, 67–77 (2011).
Yu, Z., Guo, J., Hu, M., Gao, Y. & Huang, L. Icaritin exacerbates mitophagy and synergizes with doxorubicin to induce immunogenic cell death in hepatocellular carcinoma. ACS Nano 14, 4816–4828 (2020). Co-delivery of icaritin and doxorubicin leads to ICD and triggers a robust immune response against tumours.
Bazak, R., Houri, M., El Achy, S., Kamel, S. & Refaat, T. Cancer active targeting by nanoparticles: a comprehensive review of literature. J. Cancer Res. Clin. Oncol. 141, 769–784 (2015).
Choi, H. S. et al. Design considerations for tumour-targeted nanoparticles. Nat. Nanotechnol. 5, 42–47 (2010).
Von Maltzahn, G. et al. Nanoparticles that communicate in vivo to amplify tumour targeting. Nat. Mater. 10, 545–552 (2011).
Mura, S., Nicolas, J. & Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 12, 991–1003 (2013).
Chatterjee, S. & Chi-leung HUI, P. Review of stimuli-responsive polymers in drug delivery and textile application. Molecules 24, 2547 (2019).
Wang, Y. et al. Chemotherapy-sensitized in situ vaccination for malignant osteosarcoma enabled by bioinspired calcium phosphonate nanoagents. ACS Nano 17, 6247–6260 (2023).
Gu, Z. et al. Effective combination of liposome-targeted chemotherapy and PD-L1 blockade of murine colon cancer. J. Control. Release 353, 490–506 (2023).
Liu, Y. et al. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 66, 310–324 (2018).
Kuai, R. et al. Elimination of established tumors with nanodisc-based combination chemoimmunotherapy. Sci. Adv. 4, eaao1736 (2018). A nanodisc that delivers doxorubicin triggers ICD and improves anti-PD1 therapy.
Chao, Y. et al. Localized cocktail chemoimmunotherapy after in situ gelation to trigger robust systemic antitumor immune responses. Sci. Adv. 6, eaaz4204 (2020).
Famta, P. et al. Nanocarrier-based drug delivery via cell-hitchhiking: emphasizing pharmacokinetic perspective towards taming the “big-old” tumors. J. Drug Deliv. Sci. Technol. 89, 105050 (2023).
An, M. et al. Induction of necrotic cell death and activation of STING in the tumor microenvironment via cationic silica nanoparticles leading to enhanced antitumor immunity. Nanoscale 10, 9311–9319 (2018).
Zhang, X.-D. et al. Ultrasmall glutathione-protected gold nanoclusters as next generation radiotherapy sensitizers with high tumor uptake and high renal clearance. Sci. Rep. 5, 8669 (2015).
Ni, K. et al. Nanoscale metal–organic frameworks enhance radiotherapy to potentiate checkpoint blockade immunotherapy. Nat. Commun. 9, 2351 (2018).
Lu, K. et al. Low-dose X-ray radiotherapy–radiodynamic therapy via nanoscale metal–organic frameworks enhances checkpoint blockade immunotherapy. Nat. Biomed. Eng. 2, 600–610 (2018).
Liang, G., Jin, X., Zhang, S. & Xing, D. RGD peptide-modified fluorescent gold nanoclusters as highly efficient tumor-targeted radiotherapy sensitizers. Biomaterials 144, 95–104 (2017).
Wang, K. et al. Anion receptor-mediated multicomponent synergistic self-assembly of porphyrin for efficient phototherapy to elicit tumor immunotherapy. Nano Today 46, 101579 (2022).
Chen, Z. et al. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano 12, 8633–8645 (2018).
Lan, G. et al. Nanoscale metal–organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 140, 5670–5673 (2018).
Liu, X. et al. ER-targeting PDT converts tumors into in situ therapeutic tumor vaccines. ACS Nano 16, 9240–9253 (2022).
Yue, W. et al. Checkpoint blockade and nanosonosensitizer-augmented noninvasive sonodynamic therapy combination reduces tumour growth and metastases in mice. Nat. Commun. 10, 2025 (2019).
Blum, N. T., Yildirim, A., Chattaraj, R. & Goodwin, A. P. Nanoparticles formed by acoustic destruction of microbubbles and their utilization for imaging and effects on therapy by high intensity focused ultrasound. Theranostics 7, 694 (2017).
Yildirim, A., Blum, N. T. & Goodwin, A. P. Colloids, nanoparticles, and materials for imaging, delivery, ablation, and theranostics by focused ultrasound (FUS). Theranostics 9, 2572 (2019).
Liu, X. et al. PolyTLR7/8a-conjugated, antigen-trapping gold nanorods elicit anticancer immunity against abscopal tumors by photothermal therapy-induced in situ vaccination. Biomaterials 275, 120921 (2021).
Zhang, L. et al. NIR responsive tumor vaccine in situ for photothermal ablation and chemotherapy to trigger robust antitumor immune responses. J. Nanobiotechnology 19, 142 (2021).
Huang, D. et al. In situ photothermal nano-vaccine based on tumor cell membrane-coated black phosphorus-Au for photo-immunotherapy of metastatic breast tumors. Biomaterials 289, 121808 (2022).
Hou, Q. et al. Physical & chemical microwave ablation (MWA) enabled by nonionic MWA nanosensitizers repress incomplete MWA-arised liver tumor recurrence. ACS Nano 16, 5704–5718 (2022).
Di, D.-R., He, Z.-Z., Sun, Z.-Q. & Liu, J. A new nano-cryosurgical modality for tumor treatment using biodegradable MgO nanoparticles. Nanomed. Nanotechnol. 8, 1233–1241 (2012).
Yuan, F., Zhao, G. & Panhwar, F. Enhanced killing of HepG2 during cryosurgery with Fe3O4-nanoparticle improved intracellular ice formation and cell dehydration. Oncotarget 8, 92561 (2017).
Yu, X. et al. Melittin-lipid nanoparticles target to lymph nodes and elicit a systemic anti-tumor immune response. Nat. Commun. 11, 1110 (2020). A melittin-decorated nanoparticle promotes tumour antigen release and results in systemic antitumour immune responses.
Lee, J.-j et al. Genetically engineered and self-assembled oncolytic protein nanoparticles for targeted cancer therapy. Biomaterials 120, 22–31 (2017).
Li, L. et al. Burst release of encapsulated annexin A5 in tumours boosts cytotoxic T-cell responses by blocking the phagocytosis of apoptotic cells. Nat. Biomed. Eng. 4, 1102–1116 (2020).
Demaria, S., Coleman, C. N. & Formenti, S. C. Radiotherapy: changing the game in immunotherapy. Trends Cancer 2, 286–294 (2016).
Coffman, R. L., Sher, A. & Seder, R. A. Vaccine adjuvants: putting innate immunity to work. Immunity 33, 492–503 (2010).
Shekarian, T. et al. Pattern recognition receptors: immune targets to enhance cancer immunotherapy. Ann. Oncol. 28, 1756–1766 (2017).
Galluzzi, L., Buqué, A., Kepp, O., Zitvogel, L. & Kroemer, G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 17, 97–111 (2017).
Petrizzo, A. et al. Human endogenous retrovirus reactivation: implications for cancer immunotherapy. Cancers 13, 1999 (2021).
Vasou, A., Sultanoglu, N., Goodbourn, S., Randall, R. E. & Kostrikis, L. G. Targeting pattern recognition receptors (PRR) for vaccine adjuvantation: from synthetic PRR agonists to the potential of defective interfering particles of viruses. Viruses 9, 186 (2017).
Brennan, F. R. & Dougan, G. Non-clinical safety evaluation of novel vaccines and adjuvants: new products, new strategies. Vaccine 23, 3210–3222 (2005).
Kaur, A., Baldwin, J., Brar, D., Salunke, D. B. & Petrovsky, N. Toll-like receptor (TLR) agonists as a driving force behind next-generation vaccine adjuvants and cancer therapeutics. Curr. Opin. Chem. Biol. 70, 102172 (2022).
Jacoberger-Foissac, C. et al. Liposomes as tunable platform to decipher the antitumor immune response triggered by TLR and NLR agonists. Eur. J. Pharm. Biopharm. 152, 348–357 (2020).
Pulendran, B., Arunachalam, P. & O’Hagan, D. T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Discov. 20, 454–475 (2021).
Den Brok, M. H. et al. Synergy between in situ cryoablation and TLR9 stimulation results in a highly effective in vivo dendritic cell vaccine. Cancer Res. 66, 7285–7292 (2006).
Li, Z. et al. NIR/ROS‐responsive black phosphorus QD vesicles as immunoadjuvant carrier for specific cancer photodynamic immunotherapy. Adv. Funct. Mater. 30, 1905758 (2020).
Su, T. et al. STING activation in cancer immunotherapy. Theranostics 9, 7759 (2019).
Sun, L., Wu, J., Du, F., Chen, X. & Chen, Z. J. Cyclic GMP-AMP synthase is a cytosolic DNA sensor that activates the type I interferon pathway. Science 339, 786–791 (2013).
Shae, D. et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 14, 269–278 (2019).
Dane, E. L. et al. STING agonist delivery by tumour-penetrating PEG-lipid nanodiscs primes robust anticancer immunity. Nat. Mater. 21, 710–720 (2022). Nanocarriers delivering STING-activating CDNs deep into tumour tissues lead to rejection of established tumours.
Wang, Y. et al. An amphiphilic dendrimer as a light-activable immunological adjuvant for in situ cancer vaccination. Nat. Commun. 12, 4964 (2021).
Liu, S. & Jiang, S. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today 11, 285–291 (2016).
Nogueira, S. S. et al. Polysarcosine-functionalized lipid nanoparticles for therapeutic mRNA delivery. ACS Appl. Nano Mater. 3, 10634–10645 (2020).
Nguyen, T., Avci, N. G., Shin, D. H., Martinez-Velez, N. & Jiang, H. Tune up in situ autovaccination against solid tumors with oncolytic viruses. Cancers 10, 171 (2018).
Lawler, S. E., Speranza, M.-C., Cho, C.-F. & Chiocca, E. A. Oncolytic viruses in cancer treatment: a review. JAMA Oncol. 3, 841–849 (2017).
Power, A. T. et al. Carrier cell-based delivery of an oncolytic virus circumvents antiviral immunity. Mol. Ther. 15, 123–130 (2007).
Hotte, S. J. et al. An optimized clinical regimen for the oncolytic virus PV701. Clin. Cancer Res. 13, 977–985 (2007).
Guo, Z. S., Thorne, S. H. & Bartlett, D. L. Oncolytic virotherapy: molecular targets in tumor-selective replication and carrier cell-mediated delivery of oncolytic viruses. BBA Rev. Cancer 1785, 217–231 (2008).
Willmon, C. et al. Cell carriers for oncolytic viruses: Fed Ex for cancer therapy. Mol. Ther. 17, 1667–1676 (2009).
Li, L., Liu, S., Han, D., Tang, B. & Ma, J. Delivery and biosafety of oncolytic virotherapy. Front. Oncol. 10, 475 (2020).
Gorbet, M.-J., Singh, A., Mao, C., Fiering, S. & Ranjan, A. Using nanoparticles for in situ vaccination against cancer: mechanisms and immunotherapy benefits. Int. J. Hyperthermia 37, 18–33 (2020).
Lee, K. L. et al. Combination of plant virus nanoparticle-based in situ vaccination with chemotherapy potentiates antitumor response. Nano Lett. 17, 4019–4028 (2017).
Luo, L. et al. Targeted nanoparticle‐mediated gene therapy mimics oncolytic virus for effective melanoma treatment. Adv. Funct. Mater. 28, 1800173 (2018).
Patel, R., Czapar, A. E., Fiering, S., Oleinick, N. L. & Steinmetz, N. F. Radiation therapy combined with cowpea mosaic virus nanoparticle in situ vaccination initiates immune-mediated tumor regression. ACS Omega 3, 3702–3707 (2018).
Kreitz, J. et al. Programmable protein delivery with a bacterial contractile injection system. Nature 616, 357–364 (2023).
Zhou, S., Gravekamp, C., Bermudes, D. & Liu, K. Tumour-targeting bacteria engineered to fight cancer. Nat. Rev. Cancer 18, 727–743 (2018).
Chen, Q. et al. A hybrid eukaryotic–prokaryotic nanoplatform with photothermal modality for enhanced antitumor vaccination. Adv. Mater. 32, 1908185 (2020).
Li, Y. et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post‐photothermal therapy. Small 18, 2107461 (2022).
Patel, R. B. et al. Development of an in situ cancer vaccine via combinational radiation and bacterial‐membrane‐coated nanoparticles. Adv. Mater. 31, 1902626 (2019).
Wang, D. et al. Bacterial vesicle–cancer cell hybrid membrane-coated nanoparticles for tumor specific immune activation and photothermal therapy. ACS Appl. Mater. Interfaces 12, 41138–41147 (2020).
Raza, A., Hayat, U., Rasheed, T., Bilal, M. & Iqbal, H. M. “Smart” materials-based near-infrared light-responsive drug delivery systems for cancer treatment: a review. J. Mater. Res. Technol. 8, 1497–1509 (2019).
Shahriari, M. et al. Enzyme responsive drug delivery systems in cancer treatment. J. Control. Release 308, 172–189 (2019).
Ahmed, A. & Tait, S. W. Targeting immunogenic cell death in cancer. Mol. Oncol. 14, 2994–3006 (2020).
Goel, S. et al. CDK4/6 inhibition triggers anti-tumour immunity. Nature 548, 471–475 (2017).
Garg, A. D. et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology 6, e1386829 (2017).
Adkins, I., Fucikova, J., Garg, A. D., Agostinis, P. & Špíšek, R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 3, e968434 (2014).
Wang, L. et al. An ER‐targeting iridium (III) complex that induces immunogenic cell death in non‐small‐cell lung cancer. Angew. Chem. 133, 4707–4715 (2021).
Cao, Z., Li, D., Wang, J. & Yang, X. Reactive oxygen species-sensitive polymeric nanocarriers for synergistic cancer therapy. Acta Biomater. 130, 17–31 (2021).
Gonzalez-Cao, M. et al. Human endogenous retroviruses and cancer. Cancer Biol. Med. 13, 483 (2016).
Leung, D. C. & Lorincz, M. C. Silencing of endogenous retroviruses: when and why do histone marks predominate? Trends Biochem. Sci. 37, 127–133 (2012).
Ishak, C. A., Classon, M. & De Carvalho, D. D. Deregulation of retroelements as an emerging therapeutic opportunity in cancer. Trends Cancer 4, 583–597 (2018).
Häsler, J. & Strub, K. Alu elements as regulators of gene expression. Nucleic Acids Res. 34, 5491–5497 (2006).
Giorgi, G., Virgili, M., Monti, B. & Del Re, B. Long INterspersed nuclear Elements (LINEs) in brain and non-brain tissues of the rat. Cell Tissue Res. 374, 17–24 (2018).
Roulois, D. et al. DNA-demethylating agents target colorectal cancer cells by inducing viral mimicry by endogenous transcripts. Cell 162, 961–973 (2015).
Chiappinelli, K. B. et al. Inhibiting DNA methylation causes an interferon response in cancer via dsRNA including endogenous retroviruses. Cell 162, 974–986 (2015).
Gnyszka, A., Jastrzębski, Z. & Flis, S. DNA methyltransferase inhibitors and their emerging role in epigenetic therapy of cancer. Anticancer. Res. 33, 2989–2996 (2013).
Jones, P. A., Ohtani, H., Chakravarthy, A. & De Carvalho, D. D. Epigenetic therapy in immune-oncology. Nat. Rev. Cancer 19, 151–161 (2019).
Naz, A., Cui, Y., Collins, C. J., Thompson, D. H. & Irudayaraj, J. PLGA-PEG nano-delivery system for epigenetic therapy. Biomed. Pharmacother. 90, 586–597 (2017).
Denis, I. et al. Histone deacetylase inhibitor-polymer conjugate nanoparticles for acid-responsive drug delivery. Eur. J. Med. Chem. 95, 369–376 (2015).
Zhai, Y. et al. T lymphocyte membrane-decorated epigenetic nanoinducer of interferons for cancer immunotherapy. Nat. Nanotechnol. 16, 1271–1280 (2021). T cell membrane-derived nanoparticle decorated with a lysine-specific LSD1 inhibitor activates retroviral genes and leads to strong tumour-specific immune responses.
Hammerich, L., Bhardwaj, N., Kohrt, H. E. & Brody, J. D. In situ vaccination for the treatment of cancer. Immunotherapy 8, 315–330 (2016).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Tan, S., Li, D. & Zhu, X. Cancer immunotherapy: pros, cons and beyond. Biomed. Pharmacother. 124, 109821 (2020).
Han, Y., Liu, D. & Li, L. PD-1/PD-L1 pathway: current researches in cancer. Am. J. Cancer Res. 10, 727 (2020).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98 (2016).
Krieg, C., Boyman, O., Fu, Y.-X. & Kaye, J. B and T lymphocyte attenuator regulates CD8+ T cell–intrinsic homeostasis and memory cell generation. Nat. Immunol. 8, 162–171 (2007).
Brochez, L., Chevolet, I. & Kruse, V. The rationale of indoleamine 2,3-dioxygenase inhibition for cancer therapy. Eur. J. Cancer 76, 167–182 (2017).
Ni, L. & Dong, C. New checkpoints in cancer immunotherapy. Immunol. Rev. 276, 52–65 (2017).
Ngamcherdtrakul, W. et al. In situ tumor vaccination with nanoparticle co‐delivering CpG and STAT3 siRNA to effectively induce whole‐body antitumor immune response. Adv. Mater. 33, 2100628 (2021).
Chen, Q. et al. Photothermal therapy with immune-adjuvant nanoparticles together with checkpoint blockade for effective cancer immunotherapy. Nat. Commun. 7, 13193 (2016).
Wang, C. & Steinmetz, N. F. CD47 blockade and cowpea mosaic virus nanoparticle in situ vaccination triggers phagocytosis and tumor killing. Adv. Healthc. Mater. 8, 1801288 (2019).
Han, X., Li, H., Zhou, D., Chen, Z. & Gu, Z. Local and targeted delivery of immune checkpoint blockade therapeutics. Acc. Chem. Res. 53, 2521–2533 (2020).
Le, Q.-V. et al. In situ nanoadjuvant-assembled tumor vaccine for preventing long-term recurrence. ACS Nano 13, 7442–7462 (2019).
Phuengkham, H., Song, C. & Lim, Y. T. A designer scaffold with immune nanoconverters for reverting immunosuppression and enhancing immune checkpoint blockade therapy. Adv. Mater. 31, 1903242 (2019).
Wang, C. et al. In situ activation of platelets with checkpoint inhibitors for post-surgical cancer immunotherapy. Nat. Biomed. Eng. 1, 0011 (2017).
Morad, G., Helmink, B. A., Sharma, P. & Wargo, J. A. Hallmarks of response, resistance, and toxicity to immune checkpoint blockade. Cell 184, 5309–5337 (2021).
Wang, C. et al. In situ formed reactive oxygen species-responsive scaffold with gemcitabine and checkpoint inhibitor for combination therapy. Sci. Transl. Med. 10, eaan3682 (2018).
Liu, Y. et al. Tumor microenvironment-responsive prodrug nanoplatform via co-self-assembly of photothermal agent and IDO inhibitor for enhanced tumor penetration and cancer immunotherapy. Biomaterials 242, 119933 (2020).
Ruan, H. et al. A dual‐bioresponsive drug‐delivery depot for combination of epigenetic modulation and immune checkpoint blockade. Adv. Mater. 31, 1806957 (2019).
Chen, Q. et al. In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14, 89–97 (2019). A hydrogel delivering anti-CD47 antibody blocks the ‘don’t eat me’ signal of cancer cells and increases phagocytosis of cancer cells by macrophages.
Luo, J.-Q. et al. Nanoparticle-mediated CD47-SIRPα blockade and calreticulin exposure for improved cancer chemo-immunotherapy. ACS Nano 17, 8966–8979 (2023).
Farhood, B., Najafi, M. & Mortezaee, K. CD8+ cytotoxic T lymphocytes in cancer immunotherapy: a review. J. Cell. Physiol. 234, 8509–8521 (2019).
Hoekstra, M. E., Vijver, S. V. & Schumacher, T. N. Modulation of the tumor micro-environment by CD8+ T cell-derived cytokines. Curr. Opin. Immunol. 69, 65–71 (2021).
Cheng, E. M., Tsarkovsky, N. W., Sondel, P. M. & Rakhmilevich, A. L. Interleukin-12 as an in situ cancer vaccine component: a review. Cancer Immunol. Immunother. 71, 2057–2065 (2022).
Barberio, A. E. et al. Cancer cell coating nanoparticles for optimal tumor-specific cytokine delivery. ACS Nano 14, 11238–11253 (2020).
Li, J. et al. Dual-target IL-12-containing nanoparticles enhance T cell functions for cancer immunotherapy. Cell. Immunol. 349, 104042 (2020).
Hewitt, S. L. et al. Durable anticancer immunity from intratumoral administration of IL-23, IL-36γ, and OX40L mRNAs. Sci. Transl. Med. 11, eaat9143 (2019).
Hotz, C. et al. Local delivery of mRNA-encoded cytokines promotes antitumor immunity and tumor eradication across multiple preclinical tumor models. Sci. Transl. Med. 13, eabc7804 (2021).
Li, Y. et al. Multifunctional oncolytic nanoparticles deliver self-replicating IL-12 RNA to eliminate established tumors and prime systemic immunity. Nat. Cancer 1, 882–893 (2020). LNPs delivering self-replicating RNA encoding cytokines induce potent antitumour immune responses and eradicate large established tumours.
Mestas, J. & Hughes, C. C. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Assier, E. et al. Constitutive expression of IL-2Rbeta chain and its effects on IL-2-induced vascular leak syndrome. Cytokine 32, 280–286 (2005).
Perez Horta, Z. et al. Human and murine IL2 receptors differentially respond to the human-IL2 component of immunocytokines. Oncoimmunology 8, e1238538 (2019).
Singleton, D. C., Macann, A. & Wilson, W. R. Therapeutic targeting of the hypoxic tumour microenvironment. Nat. Rev. Clin. Oncol. 18, 751–772 (2021).
Kumar, V. & Gabrilovich, D. I. Hypoxia‐inducible factors in regulation of immune responses in tumour microenvironment. Immunology 143, 512–519 (2014).
Sheu, B.-C. et al. Cytokine regulation networks in the cancer microenvironment. Front. Biosci. 13, 6255–6268 (2008).
Li, J.-Y. et al. Selective depletion of regulatory T cell subsets by docetaxel treatment in patients with nonsmall cell lung cancer. J. Immunol. Res. 2014, 286170 (2014).
Dimeloe, S. et al. Human regulatory T cells lack the cyclophosphamide‐extruding transporter ABCB 1 and are more susceptible to cyclophosphamide‐induced apoptosis. Eur. J. Immunol. 44, 3614–3620 (2014).
Zhang, L. et al. In situ formed fibrin scaffold with cyclophosphamide to synergize with immune checkpoint blockade for inhibition of cancer recurrence after surgery. Adv. Funct. Mater. 30, 1906922 (2020).
Li, Z. et al. Depletion of tumor associated macrophages enhances local and systemic platelet-mediated anti-PD-1 delivery for post-surgery tumor recurrence treatment. Nat. Commun. 13, 1845 (2022). A hydrogel delivering pexidartinib leads to depletion of TAMs and improves anti-PD1 therapy.
McKinlay, C. J. et al. Charge-altering releasable transporters (CARTs) for the delivery and release of mRNA in living animals. Proc. Natl Acad. Sci. USA 114, E448–E456 (2017).
Haabeth, O. A. W. et al. Local delivery of Ox40l, Cd80, and Cd86 mRNA kindles global anticancer immunity. Cancer Res. 79, 1624–1634 (2019).
Zhang, F. et al. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 10, 3974 (2019).
Zhang, Y. et al. Upregulation of antioxidant capacity and nucleotide precursor availability suffices for oncogenic transformation. Cell Metab. 33, 94–109.e108 (2021).
Chen, B. et al. Metabolic modulation via mTOR pathway and anti-angiogenesis remodels tumor microenvironment using PD-L1-targeting codelivery. Biomaterials 255, 120187 (2020).
Xia, C. et al. Redox-responsive nanoassembly restrained myeloid-derived suppressor cells recruitment through autophagy-involved lactate dehydrogenase A silencing for enhanced cancer immunochemotherapy. J. Control. Release 335, 557–574 (2021).
Zeng, Z. et al. Activatable polymer nanoenzymes for photodynamic immunometabolic cancer therapy. Adv. Mater. 33, 2007247 (2021).
Liu, H. et al. ADORA1 inhibition promotes tumor immune evasion by regulating the ATF3-PD-L1 axis. Cancer Cell 37, 324–339.e328 (2020).
Bergers, G. & Fendt, S.-M. The metabolism of cancer cells during metastasis. Nat. Rev. Cancer 21, 162–180 (2021).
Bloom, A. C. et al. Intratumorally delivered formulation, INT230-6, containing potent anticancer agents induces protective T cell immunity and memory. OncoImmunology 8, e1625687 (2019).
Anselmo, A. C. & Mitragotri, S. Nanoparticles in the clinic: an update. Bioeng. Transl. Med. 4, e10143 (2019).
Márquez-Rodas, I. et al. Intratumoral nanoplexed poly I:C BO-112 in combination with systemic anti–PD-1 for patients with anti–PD-1–refractory tumors. Sci. Transl. Med. 12, eabb0391 (2020).
Sabree, S., Voigt, A., Weiner, G. J. & Blackwell, S. Direct and indirect immune effects of CMP-001, a virus like particle containing a TLR9 agonist. Cancer Res. 81, 1699–1699 (2021).
Hewitt, S. L. et al. Intratumoral IL12 mRNA therapy promotes TH1 transformation of the tumor microenvironment. Clin. Cancer Res. 26, 6284–6298 (2020).
Chan, T. A. et al. Development of tumor mutation burden as an immunotherapy biomarker: utility for the oncology clinic. Ann. Oncol. 30, 44–56 (2019).
Giavridis, T. et al. CAR T cell–induced cytokine release syndrome is mediated by macrophages and abated by IL-1 blockade. Nat. Med. 24, 731–738 (2018).
Lin, M. J. et al. Cancer vaccines: the next immunotherapy frontier. Nat. Cancer 3, 911–926 (2022).
Shae, D. et al. At the bench: engineering the next generation of cancer vaccines. J. Leukoc. Biol. 108, 1435–1453 (2020).
Marabelle, A., Tselikas, L., De Baere, T. & Houot, R. Intratumoral immunotherapy: using the tumor as the remedy. Ann. Oncol. 28, xii33–xii43 (2017).
Hinohara, K. & Polyak, K. Intratumoral heterogeneity: more than just mutations. Trends Cell Biol. 29, 569–579 (2019).
Yarchoan, M., Johnson, B. A., Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat. Rev. Cancer 17, 209–222 (2017).
Buonaguro, L. & Tagliamonte, M. Selecting target antigens for cancer vaccine development. Vaccines 8, 615 (2020).
Makkouk, A. & Weiner, G. J. Cancer immunotherapy and breaking immune tolerance: new approaches to an old challenge. Cancer Res. 75, 5–10 (2015).
Min, Y. et al. Antigen-capturing nanoparticles improve the abscopal effect and cancer immunotherapy. Nat. Nanotechnol. 12, 877–882 (2017).
Ariff, B. et al. Imaging of liver cancer. World J. Gastroenterol. 15, 1289 (2009).
Oliva, M. R. & Saini, S. Liver cancer imaging: role of CT, MRI, US and PET. Cancer Imaging 4, S42 (2004).
Coulie, P. G., Van den Eynde, B. J., Van Der Bruggen, P. & Boon, T. Tumour antigens recognized by T lymphocytes: at the core of cancer immunotherapy. Nat. Rev. Cancer 14, 135–146 (2014).
Schiller, J. T. & Lowy, D. R. Vaccines to prevent infections by oncoviruses. Annu. Rev. Microbiol. 64, 23–41 (2010).
Lang, F., Schrörs, B., Löwer, M., Türeci, Ö. & Sahin, U. Identification of neoantigens for individualized therapeutic cancer vaccines. Nat. Rev. Drug Discov. 21, 261–282 (2022).
Blass, E. & Ott, P. A. Advances in the development of personalized neoantigen-based therapeutic cancer vaccines. Nat. Rev. Clin. Oncol. 18, 215–229 (2021).
Acknowledgements
M.J.M. acknowledges support from a US NIH Director’s New Innovator Award (no. DP2TR002776), a Burroughs Wellcome Fund Career Award at the Scientific Interface (CASI), the American Cancer Society (RSG-22-122-01-ET) and an NSF CAREER Award (no. CBET-2145491).
Author information
Authors and Affiliations
Contributions
N.G., M.-G.A., D.W. and M.J.M. developed the concept, researched data and wrote the article. R.E.-M. and L.X. contributed substantially to discussion of the content. R.E.-M. helped with language and figure modifications. All authors reviewed and edited the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
D.W. is named on patents that describe the use of nucleoside modified as a platform to deliver therapeutic proteins and vaccines. M.J.M, N.G., D.W. and M.-G.A. are named on patents describing the use of lipid nanoparticles and lipid compositions for nucleic acid delivery. The other authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Drug Discovery thanks Joshua Brody, Zhen Gu and Zongmin Zhao for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Adjuvant
-
A substance that enhances the immune response to an antigen with which it is mixed.
- Antigen-presenting cells
-
(APCs). Immune cells that process antigens and display their peptide fragments on the cell surface together with molecules required for T cell activation. The main APCs for T cells are dendritic cells, macrophages and B cells.
- Cytokines
-
Secreted proteins that act on specific cytokine receptors to affect cellular behaviour. Cytokines made by lymphocytes are often called lymphokines or interleukins.
- Enhanced permeability and retention effect
-
(EPR effect). An effect defined by the heightened build-up of macromolecules, including liposomes, drugs and nanoparticles, in tumours compared with normal tissues. This phenomenon occurs because the blood vessels in tumour areas are permeable and the lymphatic system is impaired.
- Immune checkpoint
-
Inhibitory regulator of the immune system that is crucial to maintain self-tolerance, prevent autoimmunity and control the duration and extent of immune response to minimize collateral tissue damage. Immune checkpoint proteins are often overexpressed on tumour cells and compromise the antitumour immune response.
- Immune escape
-
The growth and metastasis of tumours by avoiding recognition and attack by the immune system through various mechanisms.
- Immune surveillance
-
The detection and elimination of tumours by lymphocytes specific for tumour antigens.
- Pattern recognition receptors
-
Proteins capable of recognizing molecules frequently found in pathogens (pathogen-associated molecular patterns; PAMPs) or molecules released by damaged cells (damage-associated molecular patterns; DAMPs).
- Stimuli-responsive materials
-
Materials that are capable of altering their chemical and/or physical properties upon exposure to external stimuli.
- T cell exhaustion
-
The gradual loss of T cell effector function and memory characteristics that occurs under continuous antigen exposure.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Gong, N., Alameh, MG., El-Mayta, R. et al. Enhancing in situ cancer vaccines using delivery technologies. Nat Rev Drug Discov 23, 607–625 (2024). https://doi.org/10.1038/s41573-024-00974-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41573-024-00974-9
This article is cited by
-
Grain-sized moxibustion activates dendritic cells to enhance the antitumor immunity of cancer vaccines
Chinese Medicine (2025)
-
Nanomaterials-driven in situ vaccination: a novel frontier in tumor immunotherapy
Journal of Hematology & Oncology (2025)
-
Peptide hydrogel platform encapsulating manganese ions and high-density lipoprotein nanoparticle-mimicking nanovaccines for the prevention and treatment of gastric cancer
Journal of Translational Medicine (2025)
-
Nanoparticle-mediated delivery of oncolytic viral genomes: an innovative strategy for tumor-targeted immunotherapy
Cancer Nanotechnology (2025)
-
A KIF20A-based thermosensitive hydrogel vaccine effectively potentiates immune checkpoint blockade therapy for hepatocellular carcinoma
npj Vaccines (2025)