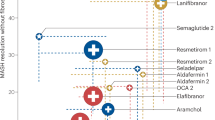
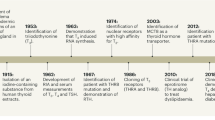
Abstract
Thyroid hormones (triiodothyronine and thyroxine) are pivotal for metabolic balance in the liver and entire body. Dysregulation of the hypothalamus–pituitary–thyroid axis can contribute to hepatic metabolic disturbances, affecting lipid metabolism, glucose regulation and protein synthesis. In addition, reductions in circulating and intrahepatic thyroid hormone concentrations increase the risk of metabolic dysfunction-associated steatotic liver disease by inducing lipotoxicity, inflammation and fibrosis. Amelioration of hepatic metabolic disease by thyroid hormones in preclinical and clinical studies has spurred the development of thyromimetics that target THRB (the predominant thyroid hormone receptor isoform in the liver) and/or the liver itself to provide more selective activation of hepatic thyroid hormone-regulated metabolic pathways while reducing thyrotoxic side effects in tissues that predominantly express THRA such as the heart and bone. Resmetirom, a liver and THRB-selective thyromimetic, recently became the first FDA-approved drug for metabolic dysfunction-associated steatohepatitis (MASH). Thus, a better understanding of the metabolic actions of thyroid hormones and thyromimetics in the liver is timely and clinically relevant. Here, we describe the roles of thyroid hormones in normal liver function and pathogenesis of MASH, as well as some potential clinical issues that might arise when treating patients with MASH with thyroid hormone supplementation or thyromimetics.
Key points
-
Hypothyroidism is associated with metabolic dysfunction-associated steatohepatitis (MASH).
-
Deiodinase 1 mRNA and protein expression and activity are downregulated as MASH progresses to cause ‘intrahepatic’ hypothyroidism.
-
Increased lipogenesis and decreased fatty acid β-oxidation cause hepatosteatosis and lipotoxicity that lead to inflammation and fibrosis in MASH.
-
Thyroid hormones increase autophagy of lipids (lipophagy), β-oxidation of fatty acids and mitochondrial turnover to reverse inflammation and fibrosis.
-
Thyroid hormones or thyromimetics are effective therapeutic agents for MASH in mouse and human studies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Roelfsema, F., Boelen, A., Kalsbeek, A. & Fliers, E. Regulatory aspects of the human hypothalamus-pituitary-thyroid axis. Best. Pract. Res. Clin. Endocrinol. Metab. 31, 487–503 (2017).
Refetoff, S. in Endotext (eds Feingold K. R. et al.) (MDText.com, 2000).
Pappa, T., Ferrara, A. M. & Refetoff, S. Inherited defects of thyroxine-binding proteins. Best. Pract. Res. Clin. Endocrinol. Metab. 29, 735–747 (2015).
Groeneweg, S., van Geest, F. S., Peeters, R. P., Heuer, H. & Visser, W. E. Thyroid hormone transporters. Endocr. Rev. 41, bnz008 (2020).
Russo, S. C., Salas-Lucia, F. & Bianco, A. C. Deiodinases and the metabolic code for thyroid hormone action. Endocrinology 162, bqab059 (2021).
Bianco, A. C. et al. Paradigms of dynamic control of thyroid hormone signaling. Endocr. Rev. 40, 1000–1047 (2019).
Feng, X., Jiang, Y., Meltzer, P. & Yen, P. M. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol. Endocrinol. 14, 947–955 (2000).
Pihlajamaki, J. et al. Thyroid hormone-related regulation of gene expression in human fatty liver. J. Clin. Endocrinol. Metab. 94, 3521–3529 (2009).
Ohba, K. et al. Desensitization and incomplete recovery of hepatic target genes after chronic thyroid hormone treatment and withdrawal in male adult mice. Endocrinology 157, 1660–1672 (2016).
de Assis, L. V. M. et al. Tuning of liver circadian transcriptome rhythms by thyroid hormone state in male mice. Sci. Rep. 14, 640 (2024).
Anselmo, J. & Chaves, C. M. Physiologic significance of epigenetic regulation of thyroid hormone target gene expression. Eur. Thyroid. J. 9, 114–123 (2020).
Darras, V. M., Houbrechts, A. M. & Van Herck, S. L. Intracellular thyroid hormone metabolism as a local regulator of nuclear thyroid hormone receptor-mediated impact on vertebrate development. Biochim. Biophys. Acta 1849, 130–141 (2015).
Rodd, C., Schwartz, H. L., Strait, K. A. & Oppenheimer, J. H. Ontogeny of hepatic nuclear triiodothyronine receptor isoforms in the rat. Endocrinology 131, 2559–2564 (1992).
Keijzer, R. et al. Expression of thyroid hormone receptors A and B in developing rat tissues; evidence for extensive posttranscriptional regulation. J. Mol. Endocrinol. 38, 523–535 (2007).
Forrest, D. & Vennstrom, B. Functions of thyroid hormone receptors in mice. Thyroid 10, 41–52 (2000).
Feng, X., Jiang, Y., Meltzer, P. & Yen, P. M. Transgenic targeting of a dominant negative corepressor to liver blocks basal repression by thyroid hormone receptor and increases cell proliferation. J. Biol. Chem. 276, 15066–15072 (2001).
Astapova, I. & Hollenberg, A. N. The in vivo role of nuclear receptor corepressors in thyroid hormone action. Biochim. Biophys. Acta 1830, 3876–3881 (2013).
Yen, P. M. Physiological and molecular basis of thyroid hormone action. Physiol. Rev. 81, 1097–1142 (2001).
Mullur, R., Liu, Y. Y. & Brent, G. A. Thyroid hormone regulation of metabolism. Physiol. Rev. 94, 355–382 (2014).
Brtko, J. Thyroid hormone and thyroid hormone nuclear receptors: history and present state of art. Endocr. Regul. 55, 103–119 (2021).
Bhat, M. K., Parkison, C., McPhie, P., Liang, C. M. & Cheng, S. Y. Conformational changes of human β1 thyroid hormone receptor induced by binding of 3,3′,5-triiodo-L-thyronine. Biochem. Biophys. Res. Commun. 195, 385–392 (1993).
Astapova, I. Role of co-regulators in metabolic and transcriptional actions of thyroid hormone. J. Mol. Endocrinol. 56, 73–97 (2016).
Liu, Y., Xia, X., Fondell, J. D. & Yen, P. M. Thyroid hormone-regulated target genes have distinct patterns of coactivator recruitment and histone acetylation. Mol. Endocrinol. 20, 483–490 (2006).
Praestholm, S. M. et al. Multiple mechanisms regulate H3 acetylation of enhancers in response to thyroid hormone. PLoS Genet. 16, e1008770 (2020).
Malik, S. et al. Structural and functional organization of TRAP220, the TRAP/mediator subunit that is targeted by nuclear receptors. Mol. Cell Biol. 24, 8244–8254 (2004).
Pandey, P. K. et al. Activation of TRAP/mediator subunit TRAP220/Med1 is regulated by mitogen-activated protein kinase-dependent phosphorylation. Mol. Cell Biol. 25, 10695–10710 (2005).
Cordeiro, A., Souza, L. L., Einicker-Lamas, M. & Pazos-Moura, C. C. Non-classic thyroid hormone signalling involved in hepatic lipid metabolism. J. Endocrinol. 216, R47–R57 (2013).
Davis, P. J., Shih, A., Lin, H. Y., Martino, L. J. & Davis, F. B. Thyroxine promotes association of mitogen-activated protein kinase and nuclear thyroid hormone receptor (TR) and causes serine phosphorylation of TR. J. Biol. Chem. 275, 38032–38039 (2000).
Gionfra, F. et al. The role of thyroid hormones in hepatocyte proliferation and liver cancer. Front. Endocrinol. 10, 532 (2019).
Tang, Q., Zeng, M., Chen, L. & Fu, N. Targeting thyroid hormone/thyroid hormone receptor axis: an attractive therapy strategy in liver diseases. Front. Pharmacol. 13, 871100 (2022).
Sinha, R. A. & Yen, P. M. Metabolic messengers: thyroid hormones. Nat. Metab. 6, 639–650 (2024).
Dittrich, R. et al. Thyroid hormone receptors and reproduction. J. Reprod. Immunol. 90, 58–66 (2011).
Selva, D. M. & Hammond, G. L. Thyroid hormones act indirectly to increase sex hormone-binding globulin production by liver via hepatocyte nuclear factor-4α. J. Mol. Endocrinol. 43, 19–27 (2009).
Shen, M. & Shi, H. Sex hormones and their receptors regulate liver energy homeostasis. Int. J. Endocrinol. 2015, 294278 (2015).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 78, 1966–1986 (2023).
Fonseca, T. L. et al. Perinatal deiodinase 2 expression in hepatocytes defines epigenetic susceptibility to liver steatosis and obesity. Proc. Natl Acad. Sci. USA 112, 14018–14023 (2015).
Fonseca, T. L. et al. Hepatic inactivation of the type 2 deiodinase confers resistance to alcoholic liver steatosis. Alcohol. Clin. Exp. Res. 43, 1376–1383 (2019).
Castillo, M. et al. Disruption of thyroid hormone activation in type 2 deiodinase knockout mice causes obesity with glucose intolerance and liver steatosis only at thermoneutrality. Diabetes 60, 1082–1089 (2011).
Fonseca, T. L., Garcia, T., Fernandes, G. W., Nair, T. M. & Bianco, A. C. Neonatal thyroxine activation modifies epigenetic programming of the liver. Nat. Commun. 12, 4446 (2021).
Hidalgo-Alvarez, J., Salas-Lucia, F., Vera Cruz, D., Fonseca, T. L. & Bianco, A. C. Localized T3 production modifies the transcriptome and promotes the hepatocyte-like lineage in iPSC-derived hepatic organoids. JCI insight 8, e173780 (2023).
Alves-Bezerra, M. & Cohen, D. E. Triglyceride metabolism in the liver. Compr. Physiol. 8, 1–8 (2017).
Bruinstroop, E. et al. Low-dose levothyroxine reduces intrahepatic lipid content in patients with type 2 diabetes mellitus and NAFLD. J. Clin. Endocrinol. Metab. 103, 2698–2706 (2018).
Kouidhi, S. & Clerget-Froidevaux, M. S. Integrating thyroid hormone signaling in hypothalamic control of metabolism: crosstalk between nuclear receptors. Int. J. Mol. Sci. 19, 2017 (2018).
López, M., Alvarez, C. V., Nogueiras, R. & Diéguez, C. Energy balance regulation by thyroid hormones at central level. Trends Mol. Med. 19, 418–427 (2013).
Ritter, M. J., Amano, I. & Hollenberg, A. N. Thyroid hormone signaling and the liver. Hepatology 72, 742–752 (2020).
Sinha, R. A. et al. Thyroid hormone stimulates hepatic lipid catabolism via activation of autophagy. J. Clin. Invest. 122, 2428–2438 (2012).
Tseng, Y. H. et al. Chromosome 19 open reading frame 80 is upregulated by thyroid hormone and modulates autophagy and lipid metabolism. Autophagy 10, 20–31 (2014).
Nelson, B. D., Luciakova, K., Li, R. & Betina, S. The role of thyroid hormone and promoter diversity in the regulation of nuclear encoded mitochondrial proteins. Biochim. Biophys. Acta 1271, 85–91 (1995).
Ramanathan, R., Patwa, S. A., Ali, A. H. & Ibdah, J. A. Thyroid hormone and mitochondrial dysfunction: therapeutic implications for metabolic dysfunction-associated steatotic liver disease (MASLD). Cells 12, 2806 (2023).
Weitzel, J. M. & Iwen, K. A. Coordination of mitochondrial biogenesis by thyroid hormone. Mol. Cell. Endocrinol. 342, 1–7 (2011).
Singh, B. K. et al. Thyroid hormone receptor and ERRα coordinately regulate mitochondrial fission, mitophagy, biogenesis, and function. Sci. Signal. 11, eaam5855 (2018).
Thakran, S. et al. Role of sirtuin 1 in the regulation of hepatic gene expression by thyroid hormone. J. Biol. Chem. 288, 807–818 (2013).
Sinha, R. A. et al. Thyroid hormone induction of mitochondrial activity is coupled to mitophagy via ROS-AMPK-ULK1 signaling. Autophagy 11, 1341–1357 (2015).
Sinha, R. A. & Yen, P. M. Thyroid hormone-mediated autophagy and mitochondrial turnover in NAFLD. Cell Biosci. 6, 46 (2016).
Lopez, D., Abisambra Socarras, J. F., Bedi, M. & Ness, G. C. Activation of the hepatic LDL receptor promoter by thyroid hormone. Biochim. Biophys. Acta 1771, 1216–1225 (2007).
Shin, D. J. & Osborne, T. F. Thyroid hormone regulation and cholesterol metabolism are connected through Sterol Regulatory Element-Binding Protein-2 (SREBP-2). J. Biol. Chem. 278, 34114–34118 (2003).
Bonde, Y. et al. Thyroid hormone reduces PCSK9 and stimulates bile acid synthesis in humans. J. Lipid Res. 55, 2408–2415 (2014).
Ness, G. C., Pendleton, L. C., Li, Y. C. & Chiang, J. Y. Effect of thyroid hormone on hepatic cholesterol 7α hydroxylase, LDL receptor, HMG-CoA reductase, farnesyl pyrophosphate synthetase and apolipoprotein A-I mRNA levels in hypophysectomized rats. Biochem. Biophys. Res. Commun. 172, 1150–1156 (1990).
Johansson, L. et al. Selective thyroid receptor modulation by GC-1 reduces serum lipids and stimulates steps of reverse cholesterol transport in euthyroid mice. Proc. Natl Acad. Sci. USA 102, 10297–10302 (2005).
Bakker, O., Hudig, F., Meijssen, S. & Wiersinga, W. M. Effects of triiodothyronine and amiodarone on the promoter of the human LDL receptor gene. Biochem. Biophys. Res. Commun. 249, 517–521 (1998).
Yap, C. S., Sinha, R. A., Ota, S., Katsuki, M. & Yen, P. M. Thyroid hormone negatively regulates CDX2 and SOAT2 mRNA expression via induction of miRNA-181d in hepatic cells. Biochem. Biophys. Res. Commun. 440, 635–639 (2013).
Davidson, N. O., Powell, L. M., Wallis, S. C. & Scott, J. Thyroid hormone modulates the introduction of a stop codon in rat liver apolipoprotein B messenger RNA. J. Biol. Chem. 263, 13482–13485 (1988).
Goldberg, I. J. et al. Thyroid hormone reduces cholesterol via a non-LDL receptor-mediated pathway. Endocrinology 153, 5143–5149 (2012).
Lammel Lindemann, J. A., Angajala, A., Engler, D. A., Webb, P. & Ayers, S. D. Thyroid hormone induction of human cholesterol 7 alpha-hydroxylase (Cyp7a1) in vitro. Mol. Cell Endocrinol. 388, 32–40 (2014).
Vatner, D. F. et al. Thyroid hormone receptor-β agonists prevent hepatic steatosis in fat-fed rats but impair insulin sensitivity via discrete pathways. Am. J. Physiol. Endocrinol. Metab. 305, E89–E100 (2013).
Nebioglu, S., Wathanaronchai, P., Nebioglu, D., Pruden, E. L. & Gibson, D. M. Mechanisms underlying enhanced glycogenolysis in livers of 3,5,3′-triiodothyronine-treated rats. Am. J. Physiol. 258, E109–E116 (1990).
McCulloch, A. J. et al. Evidence that thyroid hormones regulate gluconeogenesis from glycerol in man. Clin. Endocrinol. 19, 67–76 (1983).
Sinha, R. A., Singh, B. K. & Yen, P. M. Thyroid hormone regulation of hepatic lipid and carbohydrate metabolism. Trends Endocrinol. Metab. 25, 538–545 (2014).
Park, E. A., Song, S., Vinson, C. & Roesler, W. J. Role of CCAAT enhancer-binding protein β in the thyroid hormone and cAMP induction of phosphoenolpyruvate carboxykinase gene transcription. J. Biol. Chem. 274, 211–217 (1999).
Suh, J. H. et al. SIRT1 is a direct coactivator of thyroid hormone receptor β1 with gene-specific actions. PLoS ONE 8, e70097 (2013).
Singh, B. K. et al. Hepatic FOXO1 target genes are co-regulated by thyroid hormone via RICTOR protein deacetylation and MTORC2-AKT protein inhibition. J. Biol. Chem. 291, 198–214 (2016).
Singh, B. K. et al. FoxO1 deacetylation regulates thyroid hormone-induced transcription of key hepatic gluconeogenic genes. J. Biol. Chem. 288, 30365–30372 (2013).
Weinstein, S. P., O’Boyle, E., Fisher, M. & Haber, R. S. Regulation of GLUT2 glucose transporter expression in liver by thyroid hormone: evidence for hormonal regulation of the hepatic glucose transport system. Endocrinology 135, 649–654 (1994).
Nader, N. S. et al. Relationships between thyroid function and lipid status or insulin resistance in a pediatric population. Thyroid 20, 1333–1339 (2010).
Yan, Y. et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nat. Commun. 13, 6408 (2022).
Sokoloff, L. & Kaufman, S. Effects of thyroxin on amino acid incorporation into protein. Science 129, 569–570 (1959).
Raza, S., Rajak, S., Anjum, B. & Sinha, R. A. Molecular links between non-alcoholic fatty liver disease and hepatocellular carcinoma. Hepatoma Res. 5, 42 (2019).
Kenessey, A. & Ojamaa, K. Thyroid hormone stimulates protein synthesis in the cardiomyocyte by activating the Akt-mTOR and p70S6K pathways. J. Biol. Chem. 281, 20666–20672 (2006).
Grofte, T. et al. Hepatic amino nitrogen conversion and organ N-contents in hypothyroidism, with thyroxine replacement, and in hyperthyroid rats. J. Hepatol. 26, 409–416 (1997).
Chen, X. et al. Thyroid hormone-regulated expression of period2 promotes liver urate production. Front. Cell Dev. Biol. 9, 636802 (2021).
Araki, O., Ying, H., Zhu, X. G., Willingham, M. C. & Cheng, S. Y. Distinct dysregulation of lipid metabolism by unliganded thyroid hormone receptor isoforms. Mol. Endocrinol. 23, 308–315 (2009).
Chaves, C., Bruinstroop, E., Refetoff, S., Yen, P. M. & Anselmo, J. Increased hepatic fat content in patients with resistance to thyroid hormone beta. Thyroid 31, 1127–1134 (2021).
Laclaustra, M. et al. Impaired sensitivity to thyroid hormones is associated with diabetes and metabolic syndrome. Diabetes Care 42, 303–310 (2019).
Jornayvaz, F. R. et al. Thyroid hormone receptor-α gene knockout mice are protected from diet-induced hepatic insulin resistance. Endocrinology 153, 583–591 (2012).
Manka, P. et al. Thyroid hormone receptor alpha modulates fibrogenesis in hepatic stellate cells. Liver Int. 44, 125–138 (2024).
Kwakkel, J. et al. A novel role for the thyroid hormone-activating enzyme type 2 deiodinase in the inflammatory response of macrophages. Endocrinology 155, 2725–2734 (2014).
Fava, G. et al. Thyroid hormone inhibits biliary growth in bile duct-ligated rats by PLC/IP3/Ca2+-dependent downregulation of SRC/ERK1/2. Am. J. Physiol. Cell Physiol. 292, C1467–C1475 (2007).
Bruinstroop, E., van der Spek, A. H. & Boelen, A. Role of hepatic deiodinases in thyroid hormone homeostasis and liver metabolism, inflammation, and fibrosis. Eur. Thyroid. J. 12, e220211 (2023).
Bruinstroop, E. et al. Early induction of hepatic deiodinase type 1 inhibits hepatosteatosis during NAFLD progression. Mol. Metab. 53, 101266 (2021).
Bohinc, B. N. et al. Repair-related activation of hedgehog signaling in stromal cells promotes intrahepatic hypothyroidism. Endocrinology 155, 4591–4601 (2014).
Friesema, E. C. et al. Identification of monocarboxylate transporter 8 as a specific thyroid hormone transporter. J. Biol. Chem. 278, 40128–40135 (2003).
Friesema, E. C. et al. Mechanisms of disease: psychomotor retardation and high T3 levels caused by mutations in monocarboxylate transporter 8. Nat. Clin. Pract. Endocrinol. Metab. 2, 512–523 (2006).
Muller, J. et al. Tissue-specific alterations in thyroid hormone homeostasis in combined Mct10 and Mct8 deficiency. Endocrinology 155, 315–325 (2014).
van Geest, F. S., Gunhanlar, N., Groeneweg, S. & Visser, W. E. Monocarboxylate transporter 8 deficiency: from pathophysiological understanding to therapy development. Front. Endocrinol. 12, 723750 (2021).
Wirth, E. K., Rijntjes, E., Meyer, F., Kohrle, J. & Schweizer, U. High T3, low T4 serum levels in Mct8 deficiency are not caused by increased hepatic conversion through type I deiodinase. Eur. Thyroid. J. 4, 87–91 (2015).
Hones, G. S. et al. Cell-specific transport and thyroid hormone receptor isoform selectivity account for hepatocyte-targeted thyromimetic action of MGL-3196. Int. J. Mol. Sci. 23, 13714 (2022).
Mahdavi, M. et al. Investigating the prevalence of primary thyroid dysfunction in obese and overweight individuals: Tehran Thyroid Study. BMC Endocr. Disord. 21, 89 (2021).
Rinella, M. E. et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 79, 1542–1556 (2023).
Hutchison, A. L., Tavaglione, F., Romeo, S. & Charlton, M. Endocrine aspects of metabolic dysfunction-associated steatotic liver disease (MASLD): beyond insulin resistance. J. Hepatol. 79, 1524–1541 (2023).
Elshinshawy, S. et al. The interrelation between hypothyroidism and non-alcoholic fatty liver disease, a cross-sectional study. J. Clin. Exp. Hepatol. 13, 638–648 (2023).
Fan, H. et al. Low thyroid function is associated with an increased risk of advanced fibrosis in patients with metabolic dysfunction-associated fatty liver disease. BMC Gastroenterol. 23, 3 (2023).
Janota, B., Szczepańska, E., Adamek, B. & Janczewska, E. Hypothyroidism and non-alcoholic fatty liver disease: a coincidence or a causal relationship. World J. Hepatol. 15, 641–648 (2023).
Xie, J. et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: a comprehensive Mendelian randomization study. Hepatology 77, 949–964 (2023).
Bano, A. et al. Thyroid function and the risk of nonalcoholic fatty liver disease: the Rotterdam study. J. Clin. Endocrinol. Metab. 101, 3204–3211 (2016).
Lee, K. W. et al. Impact of hypothyroidism on the development of non-alcoholic fatty liver disease: a 4-year retrospective cohort study. Clin. Mol. Hepatol. 21, 372–378 (2015).
Mantovani, A. et al. Association between primary hypothyroidism and nonalcoholic fatty liver disease: a systematic review and meta-analysis. Thyroid 28, 1270–1284 (2018).
Chung, G. E. et al. Non-alcoholic fatty liver disease across the spectrum of hypothyroidism. J. Hepatol. 57, 150–156 (2012).
Pagadala, M. R. et al. Prevalence of hypothyroidism in nonalcoholic fatty liver disease. Dig. Dis. Sci. 57, 528–534 (2012).
Kim, D. et al. Subclinical hypothyroidism and low-normal thyroid function are associated with nonalcoholic steatohepatitis and fibrosis. Clin. Gastroenterol. Hepatol. 16, 123–131.e1 (2018).
Xu, L., Ma, H., Miao, M. & Li, Y. Impact of subclinical hypothyroidism on the development of non-alcoholic fatty liver disease: a prospective case-control study. J. Hepatol. 57, 1153–1154 (2012).
Kim, D. et al. Low thyroid function in nonalcoholic fatty liver disease is an independent predictor of all-cause and cardiovascular mortality. Am. J. Gastroenterol. 115, 1496–1504 (2020).
Wiseman, S. A., Powell, J. T., Humphries, S. E. & Press, M. The magnitude of the hypercholesterolemia of hypothyroidism is associated with variation in the low density lipoprotein receptor gene. J. Clin. Endocrinol. Metab. 77, 108–112 (1993).
Abbas, J. M., Chakraborty, J., Akanji, A. O. & Doi, S. A. Hypothyroidism results in small dense LDL independent of IRS traits and hypertriglyceridemia. Endocr. J. 55, 381–389 (2008).
Perra, A. et al. Thyroid hormone (T3) and TRβ agonist GC-1 inhibit/reverse nonalcoholic fatty liver in rats. FASEB J. 22, 2981–2989 (2008).
Zhou, J. et al. Thyroid hormone decreases hepatic steatosis, inflammation, and fibrosis in a dietary mouse model of nonalcoholic steatohepatitis. Thyroid 32, 725–738 (2022).
Sane, R., Wirth, E. K. & Kohrle, J. 3,5-T2-an endogenous thyroid hormone metabolite as promising lead substance in anti-steatotic drug development? Metabolites 12, 582 (2022).
de Lange, P. et al. Nonthyrotoxic prevention of diet-induced insulin resistance by 3,5-diiodo-l-thyronine in rats. Diabetes 60, 2730–2739 (2011).
Iannucci, L. F. et al. Metabolomic analysis shows differential hepatic effects of T2 and T3 in rats after short-term feeding with high fat diet. Sci. Rep. 7, 2023 (2017).
Ochani, S., Siddiqui, A. & Adnan, A. Adverse effects of long-term levothyroxine therapy in subclinical hypothyroidism. Ann. Med. Surg. 76, 103503 (2022).
Finan, B. et al. Chemical hybridization of glucagon and thyroid hormone optimizes therapeutic impact for metabolic disease. Cell 167, 843–857.e14 (2016).
Wu, R. et al. Conferring liver selectivity to a thyromimetic using a novel nanoparticle increases therapeutic efficacy in a diet-induced obesity animal model. PNAS Nexus 2, pgad252 (2023).
Ladenson, P. W. et al. Use of the thyroid hormone analogue eprotirome in statin-treated dyslipidemia. N. Engl. J. Med. 362, 906–916 (2010).
Sjouke, B. et al. Eprotirome in patients with familial hypercholesterolaemia (the AKKA trial): a randomised, double-blind, placebo-controlled phase 3 study. Lancet Diabetes Endocrinol. 2, 455–463 (2014).
Lammel Lindemann, J. & Webb, P. Sobetirome: the past, present and questions about the future. Expert. Opin. Ther. Targets 20, 145–149 (2016).
Sinha, R. A., Singh, B. K. & Yen, P. M. Direct effects of thyroid hormones on hepatic lipid metabolism. Nat. Rev. Endocrinol. 14, 259–269 (2018).
Cable, E. E. et al. Reduction of hepatic steatosis in rats and mice after treatment with a liver-targeted thyroid hormone receptor agonist. Hepatology 49, 407–417 (2009).
Erion, M. D. et al. Targeting thyroid hormone receptor-β agonists to the liver reduces cholesterol and triglycerides and improves the therapeutic index. Proc. Natl Acad. Sci. USA 104, 15490–15495 (2007).
Caddeo, A. et al. TG68, a novel thyroid hormone receptor-β agonist for the treatment of NAFLD. Int. J. Mol. Sci. 22, 13105 (2021).
Hu, L. et al. Discovery of highly potent and selective thyroid hormone receptor β agonists for the treatment of nonalcoholic steatohepatitis. J. Med. Chem. 66, 3284–3300 (2023).
Kowalik, M. A. et al. TRβ is the critical thyroid hormone receptor isoform in T3-induced proliferation of hepatocytes and pancreatic acinar cells. J. Hepatol. 53, 686–692 (2010).
Harrison, S. A. et al. A phase 3, randomized, controlled trial of resmetirom in NASH with liver fibrosis. N. Engl. J. Med. 390, 497–509 (2024).
Zhou, J. et al. A liver-specific thyromimetic, VK2809, decreases hepatosteatosis in glycogen storage disease type Ia. Thyroid 29, 1158–1167 (2019).
Harrison, S. A. et al. Resmetirom (MGL-3196) for the treatment of non-alcoholic steatohepatitis: a multicentre, randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 394, 2012–2024 (2019).
Hovingh, G. K. et al. Resmetirom (MGL-3196) in patients with heterozygous familial hypercholesterolemia. J. Am. Coll. Cardiol. 79, 1220–1222 (2022).
Wang, X., Wang, L., Geng, L., Tanaka, N. & Ye, B. Resmetirom ameliorates NASH-model mice by suppressing STAT3 and NF-κB signaling pathways in an RGS5-dependent manner. Int. J. Mol. Sci. 24, 5843 (2023).
Alonso-Merino, E. et al. Thyroid hormones inhibit TGF-β signaling and attenuate fibrotic responses. Proc. Natl Acad. Sci. USA 113, E3451–E3460 (2016).
Yu, G. et al. Thyroid hormone inhibits lung fibrosis in mice by improving epithelial mitochondrial function. Nat. Med. 24, 39–49 (2018).
Rosner, W., Aden, D. P. & Khan, M. S. Hormonal influences on the secretion of steroid-binding proteins by a human hepatoma-derived cell line. J. Clin. Endocrinol. Metab. 59, 806–808 (1984).
Jansen, H. I., Bruinstroop, E., Heijboer, A. C. & Boelen, A. Biomarkers indicating tissue thyroid hormone status: ready to be implemented yet? J. Endocrinol. 253, R21–R45 (2022).
Ferrara, S. J., Bourdette, D. & Scanlan, T. S. Hypothalamic-pituitary-thyroid axis perturbations in male mice by CNS-penetrating thyromimetics. Endocrinology 159, 2733–2740 (2018).
Harrison, S. A. et al. Resmetirom for nonalcoholic fatty liver disease: a randomized, double-blind, placebo-controlled phase 3 trial. Nat. Med. 29, 2919–2928 (2023).
Koulouri, O. & Gurnell, M. How to interpret thyroid function tests. Clin. Med. 13, 282–286 (2013).
Journy, N. M. Y. et al. Hyperthyroidism, hypothyroidism, and cause-specific mortality in a large cohort of women. Thyroid 27, 1001–1010 (2017).
Riis, T., Bonnema, S. J., Brix, T. H. & Folkestad, L. Hyperthyroidism and the risk of non-thyroid cancer: a Danish register-based long-term follow-up study. Eur. Thyroid. J. 13, e230181 (2024).
Sahin, T., Oral, A., Turker, F. & Kocak, E. Can hypothyroidism be a protective factor for hepatocellular carcinoma in cirrhosis? Medicine 99, e19492 (2020).
Pinter, M. et al. The impact of thyroid hormones on patients with hepatocellular carcinoma. PLoS ONE 12, e0181878 (2017).
Mishkin, S., Morris, H. P., Yalovsky, M. A. & Murthy, P. V. Inhibition of the growth of Morris hepatoma No. 44 in rats after induction of hypothyroidism: evidence that Morris hepatomas are thyroid dependent. Gastroenterology 77, 547–555 (1979).
Krashin, E., Piekielko-Witkowska, A., Ellis, M. & Ashur-Fabian, O. Thyroid hormones and cancer: a comprehensive review of preclinical and clinical studies. Front. Endocrinol. 10, 59 (2019).
Hassan, M. M. et al. Association between hypothyroidism and hepatocellular carcinoma: a case-control study in the United States. Hepatology 49, 1563–1570 (2009).
Ledda-Columbano, G. M., Perra, A., Loi, R., Shinozuka, H. & Columbano, A. Cell proliferation induced by triiodothyronine in rat liver is associated with nodule regression and reduction of hepatocellular carcinomas. Cancer Res. 60, 603–609 (2000).
Kowalik, M. A. et al. Thyroid hormone inhibits hepatocellular carcinoma progression via induction of differentiation and metabolic reprogramming. J. Hepatol. 72, 1159–1169 (2020).
Chan, I. H. & Privalsky, M. L. Thyroid hormone receptor mutants implicated in human hepatocellular carcinoma display an altered target gene repertoire. Oncogene 28, 4162–4174 (2009).
Lin, K. H., Shieh, H. Y., Chen, S. L. & Hsu, H. C. Expression of mutant thyroid hormone nuclear receptors in human hepatocellular carcinoma cells. Mol. Carcinog. 26, 53–61 (1999).
Barlow, C., Meister, B., Lardelli, M., Lendahl, U. & Vennstrom, B. Thyroid abnormalities and hepatocellular carcinoma in mice transgenic for v-erbA. EMBO J. 13, 4241–4250 (1994).
Yang, F. et al. Metabolic reprogramming and its clinical implication for liver cancer. Hepatology 78, 1602–1624 (2023).
Caddeo, A. et al. Potential use of TG68 – a novel thyromimetic – for the treatment of non-alcoholic fatty liver (NAFLD)-associated hepatocarcinogenesis. Front. Oncol. 13, 1127517 (2023).
Sabatino, L., Iervasi, G., Ferrazzi, P., Francesconi, D. & Chopra, I. J. A study of iodothyronine 5′-monodeiodinase activities in normal and pathological tissues in man and their comparison with activities in rat tissues. Life Sci. 68, 191–202 (2000).
Nappi, A., De Stefano, M. A., Dentice, M. & Salvatore, D. Deiodinases and cancer. Endocrinology 162, bqab016 (2021).
Refetoff, S., Weiss, R. E. & Usala, S. J. The syndromes of resistance to thyroid hormone. Endocr. Rev. 14, 348–399 (1993).
US National Library of Medicine. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT04173065 (2024).
US National Library of Medicine. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT05500222 (2024).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT05415722 (2023).
US National Library of Medicine. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT06168383 (2024).
US National Library of Medicine. ClinicalTrials.gov http://clinicaltrials.gov/ct2/show/NCT06342947 (2024).
Luong, X. G. et al. Regulation of gene transcription by thyroid hormone receptor β agonists in clinical development for the treatment of non-alcoholic steatohepatitis (NASH). PLoS ONE 15, e0240338 (2020).
Acknowledgements
This work is supported by Wellcome Trust/DBT India Alliance Fellowship (IA/I/16/2/502691) and SERB (CRG/2022/002149) awarded to R.A.S. and CSASI19may-0002 and NMRC/CIRG/1457/2016 to P.M.Y.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Gastroenterology & Hepatology thanks Amedeo Columbano, Simona Rapposelli and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Sinha, R.A., Bruinstroop, E. & Yen, P.M. Actions of thyroid hormones and thyromimetics on the liver. Nat Rev Gastroenterol Hepatol 22, 9–22 (2025). https://doi.org/10.1038/s41575-024-00991-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-024-00991-4
This article is cited by
-
Revisiting the relationship between thyroid function and metabolic dysfunction-associated steatotic liver disease in the era of resmetirom
Journal of Gastroenterology (2025)