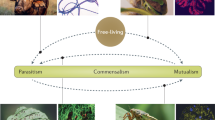
Abstract
Beneficial bacterial symbionts are widespread in insects and affect the fitness of their hosts by contributing to nutrition, digestion, detoxification, communication or protection from abiotic stressors or natural enemies. Decades of research have formed our understanding of the identity, localization and functional benefits of insect symbionts, and the increasing availability of genome sequences spanning a diversity of pathogens and beneficial bacteria now enables comparative approaches of their metabolic features and their phylogenetic affiliations, shedding new light on the origin and function of beneficial symbioses in insects. In this Review, we explore the symbionts’ metabolic traits that can provide benefits to insect hosts and discuss the evolutionary paths to the formation of host-beneficial symbiotic associations. Phylogenetic analyses and molecular studies reveal that extracellular symbioses colonizing cuticular organs or the digestive tract evolved from a broad diversity of bacterial partners, whereas intracellular beneficial symbionts appear to be restricted to a limited number of lineages within the Gram-negative bacteria and probably originated from parasitic ancestors. To unravel the general principles underlying host–symbiont interactions and recapitulate the early evolutionary steps leading towards beneficial symbioses, future efforts should aim to establish more symbiotic systems that are amenable to genetic manipulation and experimental evolution.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Drew, G. C., Stevens, E. J. & King, K. C. Microbial evolution and transitions along the parasite–mutualist continuum. Nat. Rev. Microbiol. 19, 623–638 (2021).
McFall-Ngai, M. et al. Animals in a bacterial world, a new imperative for the life sciences. Proc. Natl Acad. Sci. USA 110, 3229–3236 (2013).
Buchner, P. Endosymbiosis of Animals with Plant Microorganisms (Interscience, 1965).
Douglas, A. E. Insects and Their Beneficial Microbes (Princeton Univ. Press, 2022).
Wheeler, W. C., Whiting, M., Wheeler, Q. D. & Carpenter, J. M. The phylogeny of the extant hexapod orders. Cladistics 17, 113–169 (2001).
Cornwallis, C. K. et al. Symbioses shape feeding niches and diversification across insects. Nat. Ecol. Evol. 7, 1022–1044 (2023).
Douglas, A. E. Symbiosis as a general principle in eukaryotic evolution. Cold Spring Harb. Perspect. Biol. 6, a016113 (2014).
Husnik, F. & McCutcheon, J. P. Functional horizontal gene transfer from bacteria to eukaryotes. Nat. Rev. Microbiol. 16, 67–79 (2018).
Behmer, S. T. in Encyclopedia of Entomology (ed. Capinera, J. L.) 2646–2654 (Springer Netherlands, 2008).
Douglas, A. E. Nutritional interactions in insect–microbial symbioses: aphids and their symbiotic bacteria Buchnera. Annu. Rev. Entomol. 43, 17–37 (1998).
Douglas, A. E. The microbial dimension in insect nutritional ecology. Funct. Ecol. 23, 38–47 (2009).
Paludo, C. R. et al. Stingless bee larvae require fungal steroid to pupate. Sci. Rep. https://doi.org/10.1038/s41598-018-19583-9 (2018).
Douglas, A. E. Phloem-sap feeding by animals: problems and solutions. J. Exp. Bot. 57, 747–754 (2006).
Salem, H. & Kaltenpoth, M. Beetle–bacterial symbioses: endless forms most functional. Annu. Rev. Entomol. 67, 201–219 (2022).
Sudakaran, S., Kost, C. & Kaltenpoth, M. Symbiont acquisition and replacement as a source of ecological innovation. Trends Microbiol. 25, 375–390 (2017).
Vigneron, A. et al. Insects recycle endosymbionts when the benefit is over. Curr. Biol. 24, 2267–2273 (2014).
Andersen, S. O. Insect cuticular sclerotization: a review. Insect Biochem. Mol. Biol. 40, 166–178 (2010).
Engl, T. et al. Ancient symbiosis confers desiccation resistance to stored grain pest beetles. Mol. Ecol. 27, 2095–2108 (2018).
Anbutsu, H. et al. Small genome symbiont underlies cuticle hardness in beetles. Proc. Natl Acad. Sci. USA 114, E8382–E8391 (2017).
Zientz, E., Beyaert, N., Gross, R. & Feldhaar, H. Relevance of the endosymbiosis of Blochmannia floridanus and carpenter ants at different stages of the life cycle of the host. Appl. Environ. Microbiol. 72, 6027–6033 (2006).
Duplais, C. et al. Gut bacteria are essential for normal cuticle development in herbivorous turtle ants. Nat. Commun. 12, 676 (2021).
Jackson, R. A. H. E. L. et al. Convergent evolution of a labile nutritional symbiosis in ants. ISME J. 16, 2114–2122 (2022).
Anbutsu, H. & Fukatsu, T. in Cellular Dialogues in the Holobiont (eds Bosch, T. C. G. & Hadfield, M. G.) 201–215 (CRC, 2021).
Kanyile, S. N., Engl, T., Heddi, A. & Kaltenpoth, M. Endosymbiosis allows Sitophilus oryzae to persist in dry conditions. Front. Microbiol. 14, 1199370 (2023).
Kanyile, S. N., Engl, T. & Kaltenpoth, M. Nutritional symbionts enhance structural defence against predation and fungal infection in a grain pest beetle. J. Exp. Biol. 225, jeb243593 (2022).
Bar-Shmuel, N., Behar, A. & Segoli, M. What do we know about biological nitrogen fixation in insects? Evidence and implications for the insect and the ecosystem. Insect Sci. 27, 392–403 (2020).
Hansen, A. K., Pers, D. & Russell, J. A. in Mechanisms Underlying Microbial Symbiosis (eds Oliver, K. M. & Russell, J. A.) 161–205 (Academic–Elsevier Science, 2020).
Sabree, Z. L., Kambhampati, S. & Moran, N. A. Nitrogen recycling and nutritional provisioning by Blattabacterium, the cockroach endosymbiont. Proc. Natl Acad. Sci. USA 106, 19521–19526 (2009).
Flórez, L. V., Biedermann, P. H. W., Engl, T. & Kaltenpoth, M. Defensive symbioses of animals with prokaryotic and eukaryotic microorganisms. Nat. Prod. Rep. 32, 904–936 (2015).
Engl, T. & Kaltenpoth, M. Influence of microbial symbionts on insect pheromones. Nat. Prod. Rep. 35, 386–397 (2018).
Van Arnam, E. B., Currie, C. R. & Clardy, J. Defense contracts: molecular protection in insect–microbe symbioses. Chem. Soc. Rev. 47, 1638–1651 (2018).
Dillon, R. J., Vennard, C. T. & Charnley, A. K. Exploitation of gut bacteria in the locust. Nature 403, 851 (2000).
Ren, L., Ma, Y., Xie, M., Lu, Y. & Cheng, D. Rectal bacteria produce sex pheromones in the male oriental fruit fly. Curr. Biol. 31, 2220–2226.e4 (2021).
Wada-Katsumata, A. et al. Gut bacteria mediate aggregation in the German cockroach. Proc. Natl Acad. Sci. USA 112, 15678–15683 (2015).
Marshall, D. G. et al. Morganella morganii bacteria produces phenol as the sex pheromone of the New Zealand grass grub from tyrosine in the colleterial gland. Sci. Nat. 103, 59 (2016).
Engl, T. Sex pheromones: made with a little help from my (bacterial) friends. Curr. Biol. 31, R474–R476 (2021).
Heath, J. J., Cipollini, D. F. & Stireman, J. O. The role of carotenoids and their derivatives in mediating interactions between insects and their environment. Arthropod Plant. Interact. 7, 1–20 (2013).
Sloan, D. B. & Moran, N. A. Genome reduction and co-evolution between the primary and secondary bacterial symbionts of psyllids. Mol. Biol. Evol. 29, 3781–3792 (2012).
Moran, N. A. & Jarvik, T. Lateral transfer of genes from fungi underlies carotenoid production in aphids. Science 328, 624–627 (2010).
Nakabachi, A. et al. Defensive bacteriome symbiont with a drastically reduced genome. Curr. Biol. 23, 1478–1484 (2013).
Kellner, R. L. L. & Dettner, K. Differential efficacy of toxic pederin in deterring potential arthropod predators of Paederus (Coleoptera: Staphylinidae) offspring. Oecologia 107, 293–300 (1996).
Oliver, K. M., Degnan, P. H., Hunter, M. S. & Moran, N. A. Bacteriophages encode factors required for protection in a symbiotic mutualism. Science 325, 992–994 (2009).
Ballinger, M. J., Gawryluk, R. M. R. & Perlman, S. J. Toxin and genome evolution in a Drosophila defensive symbiosis. Genome Biol. Evol. 11, 253–262 (2019).
Ballinger, M. J. & Perlman, S. J. Generality of toxins in defensive symbiosis: ribosome-inactivating proteins and defense against parasitic wasps in Drosophila. PLoS Pathog. 13, 19 (2017).
Lindsey, A. R. I., Bhattacharya, T., Newton, I. L. G. & Hardy, R. W. Conflict in the intracellular lives of endosymbionts and viruses: a mechanistic look at Wolbachia-mediated pathogen-blocking. Viruses 10, 141 (2018).
Motta, E. V. S., Lariviere, P. J., Jones, K. R., Song, Y. L. & Moran, N. A. Type VI secretion systems promote intraspecific competition and host interactions in a bee gut symbiont. Proc. Natl Acad. Sci. USA 121, e2414882121 (2024).
Kaltenpoth, M., Gottler, W., Herzner, G. & Strohm, E. Symbiotic bacteria protect wasp larvae from fungal infestation. Curr. Biol. 15, 475–479 (2005).
Flórez, L. V. et al. Antibiotic-producing symbionts dynamically transition between plant pathogenicity and insect defensive mutualism. Nat. Commun. 8, 15172 (2017).
Flórez, L. V. et al. An antifungal polyketide associated with horizontally acquired genes supports symbiont-mediated defense in Lagria villosa beetles. Nat. Commun. 9, 2478 (2018).
Kroiss, J. et al. Symbiotic streptomycetes provide antibiotic combination prophylaxis for wasp offspring. Nat. Chem. Biol. 6, 261–263 (2010).
Piel, J. Metabolites from symbiotic bacteria. Nat. Prod. Rep. 26, 338–362 (2009).
Kaltenpoth, M. et al. Partner choice and fidelity stabilize co-evolution in a Cretaceous-age defensive symbiosis. Proc. Natl Acad. Sci. USA 111, 6359–6364 (2014).
Engl, T. et al. Evolutionary stability of antibiotic protection in a defensive symbiosis. Proc. Natl Acad. Sci. USA 115, E2020–E2029 (2018).
Oliver, K. M., Campos, J., Moran, N. A. & Hunter, M. S. Population dynamics of defensive symbionts in aphids. P. Roy. Soc. B-Biol. Sci. 275, 293–299 (2008).
Weldon, S. R., Russell, J. A. & Oliver, K. M. More is not always better: coinfections with defensive symbionts generate highly variable outcomes. Appl. Environ. Microbiol. 86, e02537-19 (2020).
Piel, J., Hofer, I. & Hui, D. Q. Evidence for a symbiosis island involved in horizontal acquisition of pederin biosynthetic capabilities by the bacterial symbiont of Paederus fuscipes beetles. J. Bacteriol. 186, 1280–1286 (2004).
Jaenike, J., Unckless, R., Cockburn, S. N., Boelio, L. M. & Perlman, S. J. Adaptation via symbiosis: recent spread of a Drosophila defensive symbiont. Science 329, 212–215 (2010).
Van Sluys, M. A. et al. Comparative genomic analysis of plant-associated bacteria. Annu. Rev. Phytopathol. 40, 169–189 (2002).
Ward, O. P. & Mooyoung, M. Enzymatic degradation of cell-wall and related plant polysaccharides. Crit. Rev. Biotechnol. 8, 237–274 (1989).
Kirsch, R. et al. Horizontal gene transfer and functional diversification of plant cell wall degrading polygalacturonases: key events in the evolution of herbivory in beetles. Insect Biochem. Mol. Biol. 52, 33–50 (2014).
Wybouw, N., Pauchet, Y., Heckel, D. G. & Van Leeuwen, T. Horizontal gene transfer contributes to the evolution of arthropod herbivory. Genome Biol. Evol. 8, 1785–1801 (2016).
Salem, H. et al. Drastic genome reduction in an herbivore’s pectinolytic symbiont. Cell 171, 1520–1531 (2017).
Kirsch, R. et al. Symbiosis and horizontal gene transfer promote herbivory in the megadiverse leaf beetles. Curr. Biol. 35, 640–654 (2025).
Kirsch, R. et al. Metabolic novelty originating from horizontal gene transfer is essential for leaf beetle survival. Proc. Natl Acad. Sci. USA 119, 9 (2022).
Brune, A. Symbiotic digestion of lignocellulose in termite guts. Nat. Rev. Microbiol. 12, 168–180 (2014).
Ceja-Navarro, J. A. et al. Gut anatomical properties and microbial functional assembly promote lignocellulose deconstruction and colony subsistence of a wood-feeding beetle. Nat. Microbiol. 4, 864–875 (2019).
Zheng, H. et al. Division of labor in honey bee gut microbiota for plant polysaccharide digestion. Proc. Natl Acad. Sci. USA 116, 25909–25916 (2019).
Engel, P., Martinson, V. G. & Moran, N. A. Functional diversity within the simple gut microbiota of the honey bee. Proc. Natl Acad. Sci. USA 109, 11002–11007 (2012).
Salem, H. et al. Symbiont digestive range reflects host plant breadth in herbivorous beetles. Curr. Biol. 30, 2875–2886 (2020).
Reis, F. et al. Bacterial symbionts support larval sap feeding and adult folivory in (semi-)aquatic reed beetles. Nat. Commun. 11, 2964 (2020).
Itoh, H., Tago, K., Hayatsu, M. & Kikuchi, Y. Detoxifying symbiosis: microbe-mediated detoxification of phytotoxins and pesticides in insects. Nat. Prod. Rep. 35, 434–454 (2018).
Dearing, M. D., Kaltenpoth, M. & Gershenzon, J. Demonstrating the role of symbionts in mediating detoxification in herbivores. Symbiosis 87, 59–66 (2022).
Motta, E. V. S. et al. Host–microbiome metabolism of a plant toxin in bees. eLife 11, e82595 (2022).
Dowd, P. F. & Shen, S. K. The contribution of symbiotic yeast to toxin resistance of the cigarette beetle (Lasioderma serricorne). Entomol. Exp. Appl. 56, 241–248 (1990).
Sato, Y. et al. Insecticide resistance by a host–symbiont reciprocal detoxification. Nat. Commun. 12, 8 (2021).
Kikuchi, Y. et al. Symbiont-mediated insecticide resistance. Proc. Natl Acad. Sci. USA 109, 8618–8622 (2012).
Ceja-Navarro, J. A. et al. Gut microbiota mediate caffeine detoxification in the primary insect pest of coffee. Nat. Commun. 6, 7618 (2015).
Shukla, S. P. & Beran, F. Gut microbiota degrades toxic isothiocyanates in a flea beetle pest. Mol. Ecol. 29, 4692–4705 (2020).
Berasategui, A. et al. Gut microbiota of the pine weevil degrades conifer diterpenes and increases insect fitness. Mol. Ecol. 26, 4099–4110 (2017).
Nikoh, N., Hosokawa, T., Oshima, K., Hattori, M. & Fukatsu, T. Reductive evolution of bacterial genome in insect gut environment. Genome Biol. Evol. 3, 702–714 (2011).
Welte, C. U. et al. Plasmids from the gut microbiome of cabbage root fly larvae encode SaxA that catalyses the conversion of the plant toxin 2-phenylethyl isothiocyanate. Environ. Microbiol. 18, 1379–1390 (2016).
Ben-Yosef, M., Pasternak, Z., Jurkevitch, E. & Yuval, B. Symbiotic bacteria enable olive fly larvae to overcome host defences. R. Soc. Open. Sci. 2, 150170 (2015).
Liu, F. H. et al. Symbiotic microbes aid host adaptation by metabolizing a deterrent host pine carbohydrate d-pinitol in a beetle–fungus invasive complex. Sci. Adv. 8, 14 (2022).
Li, H., Young, S. E., Poulsen, M. & Currie, C. R. Symbiont-mediated digestion of plant biomass in fungus-farming insects. Annu. Rev. Entomol. 66, 297–316 (2021).
Martiarena, M. J. S., Deveau, A., Montoya, Q. V., Flórez, L. V. & Rodrigues, A. The hyphosphere of leaf-cutting ant cultivars is enriched with helper bacteria. Microb. Ecol. 86, 1773–1788 (2023).
Pinto-Tomás, A. A. et al. Symbiotic nitrogen fixation in the fungus gardens of leaf-cutter ants. Science 326, 1120–1123 (2009).
Anderson, K. E., Sheehan, T. H., Eckholm, B. J., Mott, B. M. & DeGrandi-Hoffman, G. An emerging paradigm of colony health: microbial balance of the honey bee and hive (Apis mellifera). Insect Soc. 58, 431–444 (2011).
Grubbs, K. J. et al. Cycloheximide-producing Streptomyces associated with Xyleborinus saxesenii and Xyleborus affinis fungus-farming ambrosia beetles. Front. Microbiol. 11, 562140 (2020).
Pessotti, Rd. C. et al. Multiple lineages of Streptomyces produce antimicrobials within passalid beetle galleries across eastern North America. eLife 10, e65091 (2021).
Shukla, S. P. et al. Microbiome-assisted carrion preservation aids larval development in a burying beetle. Proc. Natl Acad. Sci. USA 115, 11274–11279 (2018).
Douglas, A. E. Housing microbial symbionts: evolutionary origins and diversification of symbiotic organs in animals. Philos. Trans. R. Soc. B 375, 20190603 (2020).
Chomicki, G., Werner, G. D. A., West, S. A. & Kiers, E. T. Compartmentalization drives the evolution of symbiotic cooperation. Philos. Trans. R. Soc. B 375, 20190602 (2020).
Hong, S., Sun, Y., Sun, D. & Wang, C. Microbiome assembly on Drosophila body surfaces benefits the flies to combat fungal infections. iScience 25, 104408 (2022).
Currie, C. R., Poulsen, M., Mendenhall, J., Boomsma, J. J. & Billen, J. Coevolved crypts and exocrine glands. Science 311, 81–83 (2006).
Goettler, W., Kaltenpoth, M., McDonald, S. & Strohm, E. Comparative morphology of the symbiont cultivation glands in the antennae of female digger wasps of the genus Philanthus (Hymenoptera: Crabronidae). Front. Physiol. 13, 815494 (2022).
Janke, R. S. et al. Bacterial ectosymbionts in cuticular organs chemically protect a beetle during molting stages. ISME J. 16, 2691–2701 (2022).
Worsley, S. F. et al. Competition-based screening helps to secure the evolutionary stability of a defensive microbiome. BMC Biol 19, 205 (2021).
Engel, P. & Moran, N. A. The gut microbiota of insects—diversity in structure and function. FEMS Microbiol. Rev. 37, 699–735 (2013).
Potrikus, C. J. & Breznak, J. A. Gut bacteria recycle uric acid nitrogen in termites: a strategy for nutrient conservation. Proc. Natl Acad. Sci. USA 78, 4601–4605 (1981).
Salem, H. et al. Vitamin supplementation by gut symbionts ensures metabolic homeostasis in an insect host. P. Roy. Soc. B-Biol. Sci. 281, 20141838 (2014).
Koch, H. & Schmid-Hempel, P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl Acad. Sci. USA 108, 19288–19292 (2011).
Onchuru, T. O., Martinez, A. J. & Kaltenpoth, M. The cotton stainer’s gut microbiota suppresses infection of a co-transmitted trypanosomatid parasite. Mol. Ecol. 27, 3408–3419 (2018).
Hammer, T. J., Janzen, D. H., Hallwachs, W., Jaffe, S. P. & Fierer, N. Caterpillars lack a resident gut microbiome. Proc. Natl Acad. Sci. USA 114, 9641–9646 (2017).
Hammer, T. J., Sanders, J. G. & Fierer, N. Not all animals need a microbiome. FEMS Microbiol. Lett. 366, fnz117 (2019).
Girard, M., Luis, P., Moro, C. V. & Minard, G. Crosstalk between the microbiota and insect postembryonic development. Trends Microbiol. 31, 181–196 (2022).
Kikuchi, Y., Hosokawa, T. & Fukatsu, T. Insect–microbe mutualism without vertical transmission: a stinkbug acquires a beneficial gut symbiont from the environment every generation. Appl. Environ. Microbiol. 73, 4308–4316 (2007).
Salem, H., Florez, L., Gerardo, N. & Kaltenpoth, M. An out-of-body experience: the extracellular dimension for the transmission of mutualistic bacteria in insects. P. Roy. Soc. B-Biol. Sci. 282, 20142957 (2015).
Onchuru, T. O., Javier Martinez, A., Ingham, C. S. & Kaltenpoth, M. Transmission of mutualistic bacteria in social and gregarious insects. Curr. Opin. Insect Sci. 28, 50–58 (2018).
Moran, N. A., Ochman, H. & Hammer, T. J. Evolutionary and ecological consequences of gut microbial communities. Annu. Rev. Ecol. Evol. Syst. 50, 451–475 (2019).
Schmidt, K. & Engel, P. Mechanisms underlying gut microbiota–host interactions in insects. J. Exp. Biol. 224, jeb207696 (2021).
Ganesan, R., Wierz, J., Kaltenpoth, M. & Flórez, L. V. How it all begins: bacterial factors mediating the colonization of invertebrate hosts by beneficial symbionts. Microbiol. Mol. Biol. Rev. 86, e0012621 (2022).
Chen, J. Z., Kwong, Z., Gerardo, N. M. & Vega, N. M. Ecological drift during colonization drives within-host and between-host heterogeneity in an animal-associated symbiont. PLoS Biol. 22, 25 (2024).
Itoh, H. et al. Host–symbiont specificity determined by microbe–microbe competition in an insect gut. Proc. Natl Acad. Sci. USA 116, 22673–22682 (2019).
Kwong, W. K. & Moran, N. A. Gut microbial communities of social bees. Nat. Rev. Microbiol. 14, 374–384 (2016).
Johnston, P. R., Paris, V. & Rolff, J. Immune gene regulation in the gut during metamorphosis in a holo- versus a hemimetabolous insect. Philos. Trans. R. Soc. B 374, 20190073 (2019).
Ohbayashi, T. et al. Insect’s intestinal organ for symbiont sorting. Proc. Natl Acad. Sci. USA 112, E5179–E5188 (2015).
Lanan, M. C., Rodrigues, P. A. P., Agellon, A., Jansma, P. & Wheeler, D. E. A bacterial filter protects and structures the gut microbiome of an insect. ISME J. 10, 1866–1876 (2016).
Fukumori, K. et al. Evolutionary dynamics of host organs for microbial symbiosis in tortoise leaf beetles (Coleoptera: Chrysomelidae: Cassidinae). mBio 13, e0369121 (2022).
Matsuura, Y. et al. Evolution of symbiotic organs and endosymbionts in lygaeid stinkbugs. ISME J. 6, 397–409 (2012).
Oishi, S., Moriyama, M., Koga, R. & Fukatsu, T. Morphogenesis and development of midgut symbiotic organ of the stinkbug Plautia stali (Hemiptera: Pentatomidae). Zool. Lett. 5, 16 (2019).
Kikuchi, Y., Ohbayashi, T., Jang, S. & Mergaert, P. Burkholderia insecticola triggers midgut closure in the bean bug Riptortus pedestris to prevent secondary bacterial infections of midgut crypts. ISME J. 14, 1627–1638 (2020).
Dale, C., Young, S. A., Haydon, D. T. & Welburn, S. C. The insect endosymbiont Sodalis glossinidius utilizes a type III secretion system for cell invasion. Proc. Natl Acad. Sci. USA 98, 1883–1888 (2001).
Maire, J. et al. Spatial and morphological reorganization of endosymbiosis during metamorphosis accommodates adult metabolic requirements in a weevil. Proc. Natl Acad. Sci. USA 117, 19347–19358 (2020).
Wilkes, T. E. et al. The draft genome sequence of Arsenophonus nasoniae, son-killer bacterium of Nasonia vitripennis, reveals genes associated with virulence and symbiosis. Insect Mol. Biol. 19, 59–73 (2010).
Wierz, J. C. et al. Intracellular symbiont Symbiodolus is vertically transmitted and widespread across insect orders. ISME J. 18, 13 (2024).
Schepers, M. J., Yelland, J. N., Moran, N. A. & Taylor, D. W. Isolation of the Buchnera aphidicola flagellum basal body complexes from the Buchnera membrane. PLoS ONE 16, 10 (2021).
Richter, D. J. & Levin, T. C. The origin and evolution of cell-intrinsic antibacterial defenses in eukaryotes. Curr. Opin. Genet. Dev. 58–59, 111–122 (2019).
Krakauer, T. Inflammasomes, autophagy, and cell death: the trinity of innate host defense against intracellular bacteria. Mediators Inflamm. 2019, 2471215 (2019).
Tam, J. C. H. & Jacques, D. A. Intracellular immunity: finding the enemy within—how cells recognize and respond to intracellular pathogens. J. Leukoc. Biol. 96, 233–244 (2014).
Jo, Y. H. et al. Autophagy in Tenebrio molitor immunity: conserved antimicrobial functions in insect defenses. Front. Immunol. 12, 667664 (2021).
Steinert, S. & Levashina, E. A. Intracellular immune responses of dipteran insects. Immunol. Rev. 240, 129–140 (2011).
Brenner, A. E., Muñoz-Leal, S., Sachan, M., Labruna, M. B. & Raghavan, R. Coxiella burnetii and related tick endosymbionts evolved from pathogenic ancestors. Genome Biol. Evol. 13, evab108 (2021).
Dharamshi, J. E. et al. Gene gain facilitated endosymbiotic evolution of Chlamydiae. Nat. Microbiol. 8, 40–54 (2023).
Stelzner, K., Vollmuth, N. & Rudel, T. Intracellular lifestyle of Chlamydia trachomatis and host–pathogen interactions. Nat. Rev. Microbiol. 21, 448–462 (2023).
Van Schaik, E. J., Chen, C., Mertens, K., Weber, M. M. & Samuel, J. E. Molecular pathogenesis of the obligate intracellular bacterium Coxiella burnetii. Nat. Rev. Microbiol. 11, 561–573 (2013).
Voss, O. H. & Rahman, M. S. Rickettsia–host interaction: strategies of intracytosolic host colonization. Pathog. Dis. 79, ftab015 (2021).
Feng, H. et al. Trading amino acids at the aphid–Buchnera symbiotic interface. Proc. Natl Acad. Sci. USA 116, 16003–16011 (2019).
Costa, H. S., Westcot, D. M., Ullman, D. E. & Johnson, M. W. Ultrastructure of the endosymbionts of the whitefly, Bemisia tabaci and Trialeurodes vaporariorum. Protoplasma 176, 106–115 (1993).
Wang, Y.-B. et al. Autophagy regulates whitefly–symbiont metabolic interactions. Appl. Environ. Microbiol. 88, e02089–02021 (2022).
Deehan, M., Lin, W., Blum, B., Emili, A. & Frydman, H. Intracellular density of Wolbachia is mediated by host autophagy and the bacterial cytoplasmic incompatibility gene cifB in a cell type-dependent manner in Drosophila melanogaster. mBio 12, e02205-20 (2021).
Aguilar, C., Mano, M. & Eulalio, A. microRNAs at the host–bacteria interface: host defense or bacterial offense. Trends Microbiol. 27, 206–218 (2019).
Martyn, J. E., Gomez-Valero, L. & Buchrieser, C. The evolution and role of eukaryotic-like domains in environmental intracellular bacteria: the battle with a eukaryotic cell. FEMS Microbiol. Rev. 46, fuac012 (2022).
LePage, D. P. et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature 543, 243 (2017).
Bordenstein, S. R. & Bordenstein, S. R. Eukaryotic association module in phage WO genomes from Wolbachia. Nat. Commun. 7, 13155 (2016).
Hussain, M., Frentiu, F. D., Moreira, L. A., O’Neill, S. L. & Asgari, S. Wolbachia uses host microRNAs to manipulate host gene expression and facilitate colonization of the dengue vector Aedes aegypti. Proc. Natl Acad. Sci. USA 108, 9250–9255 (2011).
Bublitz, D. A. C. et al. Peptidoglycan production by an insect–bacterial mosaic. Cell 179, 703–712.e7 (2019).
Sun, X. et al. A novel microRNA regulates cooperation between symbiont and a laterally acquired gene in the regulation of pantothenate biosynthesis within Bemisia tabaci whiteflies. Mol. Ecol. 31, 2611–2624 (2022).
Dale, C., Plague, G. R., Wang, B., Ochman, H. & Moran, N. A. Type III secretion systems and the evolution of mutualistic endosymbiosis. Proc. Natl Acad. Sci. USA 99, 12397–12402 (2002).
Chomicki, G., Kiers, E. T. & Renner, S. S. The evolution of mutualistic dependence. Annu. Rev. Ecol. Evol. Syst. 51, 409–432 (2020).
Frank, S. A. Models of symbiosis. Am. Nat. 150, S80–S99 (1997).
Su, Y. et al. Rational engineering of a synthetic insect–bacterial mutualism. Curr. Biol. 32, 3925–3938.e6 (2022).
Koga, R. et al. Single mutation makes Escherichia coli an insect mutualist. Nat. Microbiol. 7, 1141–1150 (2022).
Durvasula, R. V., Sundaram, R. K., Cordon-Rosales, C., Pennington, P. & Beard, C. B. in Insect Symbiosis (eds Bourtzis, K. & Miller, T. A.) 83–96 (CRC, 2003).
Currie, C. R., Scott, J. A., Summerbell, R. C. & Malloch, D. Fungus-growing ants use antibiotic-producing bacteria to control garden parasites. Nature 398, 701–704 (1999).
Evans, J. D. & Lopez, D. L. Bacterial probiotics induce an immune response in the honeybee (Hymenoptera: Apidae). J. Econ. Entomol. 97, 752–756 (2004).
Consuegra, J. et al. Metabolic cooperation among commensal bacteria supports Drosophila juvenile growth under nutritional stress. Iscience 23, 41 (2020).
Zheng, H., Powell, J. E., Steele, M. I., Dietrich, C. & Moran, N. A. Honeybee gut microbiota promotes host weight gain via bacterial metabolism and hormonal signaling. Proc. Natl Acad. Sci. USA 114, 4775–4780 (2017).
Shao, Y. et al. Symbiont-derived antimicrobials contribute to the control of the lepidopteran gut microbiota. Cell Chem. Biol. 24, 66–75 (2017).
Berasategui, A. et al. Symbiont genomic features and localization in the bean beetle Callosobruchus maculatus. Appl. Environ. Microbiol. 87, e0021221 (2021).
Miller, D. L., Smith, E. A. & Newton, I. L. G. A bacterial symbiont protects honey bees from fungal disease. mBio 12, e0050321 (2021).
Kaltenpoth, M. & Flórez, L. V. Versatile and dynamic symbioses between insects and Burkholderia bacteria. Annu. Rev. Entomol. 65, 145–170 (2020).
Sudakaran, S., Retz, F., Kikuchi, Y., Kost, C. & Kaltenpoth, M. Evolutionary transition in symbiotic syndromes enabled diversification of phytophagous insects on an imbalanced diet. ISME J. 9, 2587–2604 (2015).
Bonilla-Rosso, G. & Engel, P. Functional roles and metabolic niches in the honey bee gut microbiota. Curr. Opin. Microbiol. 43, 69–76 (2018).
Piel, J. A polyketide synthase–peptide synthetase gene cluster from an uncultured bacterial symbiont of Paederus beetles. Proc. Natl Acad. Sci. USA 99, 14002–14007 (2002).
Mukherjee, K. et al. Galleria mellonella as a model system for studying Listeria pathogenesis. Appl. Environ. Microbiol. 76, 310–317 (2010).
Matsuura, Y. et al. Recurrent symbiont recruitment from fungal parasites in cicadas. Proc. Natl Acad. Sci. USA 115, E5970–E5979 (2018).
Everett, K. D. E., Thao, M. L., Horn, M., Dyszynski, G. E. & Baumann, P. Novel chlamydiae in whiteflies and scale insects: endosymbionts ‘Candidatus Fritschea bemisiae’ strain Falk and ‘Candidatus Fritschea eriococci’ strain Elm. Int. J. Syst. Evol. Microbiol. 55, 1581–1587 (2005).
Nechitaylo, T. Y. et al. Incipient genome erosion and metabolic streamlining for antibiotic production in a defensive symbiont. Proc. Natl Acad. Sci. USA 118, e2023047118 (2021).
Itoh, H. et al. Infection dynamics of insecticide-degrading symbionts from soil to insects in response to insecticide spraying. ISME J. 12, 909–920 (2018).
Hosokawa, T. et al. Obligate bacterial mutualists evolving from environmental bacteria in natural insect populations. Nat. Microbiol. 1, 15011 (2016).
McCutcheon, J. P., Boyd, B. M. & Dale, C. The life of an insect endosymbiont from the cradle to the grave. Curr. Biol. 29, R485–R495 (2019).
Heckel, D. G. How do toxins from Bacillus thuringiensis kill insects? An evolutionary perspective. Arch. Insect Biochem. Physiol. 104, 12 (2020).
Nielsen-LeRoux, C., Gaudriault, S., Ramarao, N., Lereclus, D. & Givaudan, A. How the insect pathogen bacteria Bacillus thuringiensis and Xenorhabdus/Photorhabdus occupy their hosts. Curr. Opin. Microbiol. 15, 220–231 (2012).
de Bekker, C., Beckerson, W. C. & Elya, C. Mechanisms behind the madness: how do zombie-making fungal entomopathogens affect host behavior to increase transmission? mBio 12, 15 (2021).
Laukaitis, H. J. & Macaluso, K. R. Unpacking the intricacies of Rickettsia–vector interactions. Trends Parasitol. 37, 734–746 (2021).
Trivellone, V. & Dietrich, C. H. Evolutionary diversification in insect vector–Phytoplasma–plant associations. Ann. Entomol. Soc. Am. 114, 137–150 (2020).
Manzano-Marín, A. et al. Serial horizontal transfer of vitamin-biosynthetic genes enables the establishment of new nutritional symbionts in aphids’ di-symbiotic systems. ISME J. 14, 259–273 (2020).
Landmann, F. The Wolbachia endosymbionts. Microbiol. Spectr. https://doi.org/10.1128/microbiolspec.bai-0018-2019 (2019).
Hosokawa, T., Koga, R., Kikuchi, Y., Meng, X. Y. & Fukatsu, T. Wolbachia as a bacteriocyte-associated nutritional mutualist. Proc. Natl Acad. Sci. USA 107, 769–774 (2010).
Moriyama, M., Nikoh, N., Hosokawa, T. & Fukatsu, T. Riboflavin provisioning underlies Wolbachia’s fitness contribution to its insect host. mBio 6, e01732-15 (2015).
Dobson, S. L., Marsland, E. J. & Rattanadechakul, W. Mutualistic Wolbacbia infection in Aedes albopictus: accelerating cytoplasmic drive. Genetics 160, 1087–1094 (2002).
Zug, R. & Hammerstein, P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biol. Rev. 90, 89–111 (2015).
Egan, A. J. F., Errington, J. & Vollmer, W. Regulation of peptidoglycan synthesis and remodelling. Nat. Rev. Microbiol. 18, 446–460 (2020).
Wang, X. L., Zhang, Y. Q., Zhang, R. & Zhang, J. H. The diversity of pattern recognition receptors (PRRs) involved with insect defense against pathogens. Curr. Opin. Insect Sci. 33, 105–110 (2019).
Kim, S. J., Chang, J. & Singh, M. Peptidoglycan architecture of Gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta 1848, 350–362 (2015).
Maire, J., Vincent-Monégat, C., Masson, F., Zaidman-Rémy, A. & Heddi, A. An IMD-like pathway mediates both endosymbiont control and host immunity in the cereal weevil Sitophilus spp. Microbiome 6, 6 (2018).
Liegeois, S. & Ferrandon, D. Sensing microbial infections in the Drosophila melanogaster genetic model organism. Immunogenetics 74, 35–62 (2022).
Vorburger, C. Defensive symbionts and the evolution of parasitoid host specialization. Annu. Rev. Entomol. 67, 329–346 (2022).
Misof, B. et al. Phylogenomics resolves the timing and pattern of insect evolution. Science 346, 763–767 (2014).
Zhang, Z.-Q. Animal biodiversity: an outline of higher-level classification and survey of taxonomic richness. Zootaxa 3703, 17–26 (2013).
Asnicar, F. et al. Precise phylogenetic analysis of microbial isolates and genomes from metagenomes using PhyloPhlAn 3.0. Nat. Commun. 11, 2500 (2020).
Janson, E. M., Stireman, J. O., Singer, M. S. & Abbot, P. Phytophagous insect–microbe mutualisms and adaptive evolutionary diversification. Evolution 62, 997–1012 (2008).
Moran, N. A. Symbiosis as an adaptive process and source of phenotypic complexity. Proc. Natl Acad. Sci. USA 104, 8627–8633 (2007).
Bright, M. & Bulgheresi, S. A complex journey: transmission of microbial symbionts. Nat. Rev. Microbiol. 8, 218–230 (2010).
Perreau, J. & Moran, N. A. Genetic innovations in animal–microbe symbioses. Nat. Rev. Genet. 23, 23–39 (2022).
Moran, N. A. & Degnan, P. H. Functional genomics of Buchnera and the ecology of aphid hosts. Mol. Ecol. 15, 1251–1261 (2006).
Tamas, I. et al. 50 million years of genomic stasis in endosymbiotic bacteria. Science 296, 2376–2379 (2002).
Wierz, J. C., Gimmel, M. L., Huthmacher, S., Engl, T. & Kaltenpoth, M. Evolutionary history of tyrosine-supplementing endosymbionts in pollen-feeding beetles. ISME J. 18, 16 (2024).
Bennett, G. M. & Moran, N. A. Heritable symbiosis: the advantages and perils of an evolutionary rabbit hole. Proc. Natl Acad. Sci. USA 112, 10169–10176 (2015).
Acknowledgements
The authors acknowledge financial support from the Max Planck Society and the European Research Council through a Consolidator Grant to M.K. (ERC CoG 819585 ‘SYMBeetle’) and from the Novo Nordisk Foundation through a postdoctoral research grant to L.V.F. (NNF20OC0064385).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Peer review
Peer review information
Nature Reviews Microbiology thanks Nancy Moran and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kaltenpoth, M., Flórez, L.V., Vigneron, A. et al. Origin and function of beneficial bacterial symbioses in insects. Nat Rev Microbiol (2025). https://doi.org/10.1038/s41579-025-01164-z
Accepted:
Published:
DOI: https://doi.org/10.1038/s41579-025-01164-z