Abstract
Individuals who are diagnosed with amyotrophic lateral sclerosis (ALS) today face the same historically intransigent problem that has existed since the initial description of the disease in the 1860s — a lack of effective therapies. In part, the development of new treatments has been hampered by an imperfect understanding of the biological processes that trigger ALS and promote disease progression. Advances in our understanding of these biological processes, including the causative genetic mutations, and of the influence of environmental factors have deepened our appreciation of disease pathophysiology. The consequent identification of pathogenic targets means that the introduction of effective therapies is becoming a realistic prospect. Progress in precision medicine, including genetically targeted therapies, will undoubtedly change the natural history of ALS. The evolution of clinical trial designs combined with improved methods for patient stratification will facilitate the translation of novel therapies into the clinic. In addition, the refinement of emerging biomarkers of therapeutic benefits is critical to the streamlining of care for individuals. In this Review, we synthesize these developments in ALS and discuss the further developments and refinements needed to accelerate the introduction of effective therapeutic approaches.
Key points
-
The development of effective treatments for amyotrophic lateral sclerosis (ALS) has been limited by a lack of comprehensive understanding of the biological processes that trigger the disease and promote progression.
-
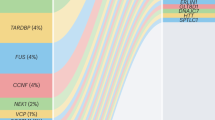
Causative genetic mutations have been identified, many of which are linked to RNA function and metabolism.
-
Disease heterogeneity suggests that a precision medicine paradigm incorporating extensive phenotypic and genotypic information will be required to realize effective therapy and improve the outcomes for individual patients with ALS.
-
The repurposing of drugs with established safety profiles from their use in other human diseases is a new approach to therapeutic discovery in ALS.
-
Enhanced clinical trial designs, including multi-arm, multi-stage platform trials, that incorporate biomarkers of treatment responses will accelerate drug discovery and increase trial participation.
-
Improved patient stratification and patient-reported outcome measures, including home assessments, will improve the reliability and sensitivity of trial endpoints.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
209,00 € per year
only 17,42 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Alzheimer’s Disease International. World Alzheimer report 2019: attitudes to dementia (ADI, 2019).
Hurd, M. D., Martorell, P., Delavande, A., Mullen, K. J. & Langa, K. M. Monetary costs of dementia in the United States. N. Engl. J. Med. 368, 1326–1334 (2013).
World Health Organization. Global action plan on the public health response to dementia 2017–2025 (WHO, 2017).
Hebert, L. E., Weuve, J., Scherr, P. A. & Evans, D. A. Alzheimer disease in the United States (2010–2050) estimated using the 2010 census. Neurology 80, 1778 (2013).
Huynh, W. et al. The impact of cognitive and behavioral impairment in amyotrophic lateral sclerosis. Expert Rev. Neurother. 20, 281–293 (2020).
Kiernan, M. C. et al. Amyotrophic lateral sclerosis. Lancet 377, 942–955 (2011).
Hardiman, O., van den Berg, L. H. & Kiernan, M. C. Clinical diagnosis and management of amyotrophic lateral sclerosis. Nat. Rev. Neurol. 7, 639 (2011).
Westeneng, H.-J. et al. Prognosis for patients with amyotrophic lateral sclerosis: development and validation of a personalised prediction model. Lancet Neurol. 17, 423–433 (2018).
Bedlack, R. S., Pastula, D., Welsh, E., Pulley, D. & Cudkowicz, M. E. Scrutinizing enrollment in ALS clinical trials: room for improvement? Amyotroph. Lateral Scler. 9, 257–265 (2008).
van den Berg, L. H. et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology 92, e1610–e1623 (2019).
Shefner, J. M. et al. A proposal for new diagnostic criteria for ALS. Clin. Neurophysiol. 113, 1975–1978 (2020).
Dharmadasa, T., Matamala, J. M., Howells, J., Vucic, S. & Kiernan, M. C. Early focality and spread of cortical dysfunction in amyotrophic lateral sclerosis: a regional study across the motor cortices. Clin. Neurophysiol. 131, 958–966 (2020).
Vucic, S., Rothstein, J. D. & Kiernan, M. C. Advances in treating amyotrophic lateral sclerosis: insights from pathophysiological studies. Trends Neurosci. 37, 433–442 (2014).
Swash, M. et al. Occasional essay: upper motor neuron syndrome in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 91, 227–234 (2020).
Huynh, W. et al. Assessment of the upper motor neuron in amyotrophic lateral sclerosis. Clin. Neurophysiol. 127, 2643–2660 (2016).
Simon, N. G. et al. Quantifying disease progression in amyotrophic lateral sclerosis. Ann. Neurol. 76, 643–657 (2014).
de Carvalho, M., Kiernan, M. C. & Swash, M. Fasciculation in amyotrophic lateral sclerosis: origin and pathophysiological relevance. J. Neurol. Neurosurg. Psychiatry 88, 773–779 (2017).
Al-Chalabi, A. & Hardiman, O. The epidemiology of ALS: a conspiracy of genes, environment and time. Nat. Rev. Neurol. 9, 617 (2013).
Turner, M. R. et al. Genetic screening in sporadic ALS and FTD. J. Neurol. Neurosurg. Psychiatry 88, 1042 (2017).
Blair, I. P. et al. FUS mutations in amyotrophic lateral sclerosis: clinical, pathological, neurophysiological and genetic analysis. J. Neurol. Neurosurg. Psychiatry 81, 639–645 (2010).
Williams, K. L. et al. Pathophysiological insights into ALS with C9ORF72 expansions. J. Neurol. Neurosurg. Psychiatry 84, 931–935 (2013).
Brown, R. H. & Al-Chalabi, A. Amyotrophic lateral sclerosis. N. Engl. J. Med. 377, 162–172 (2017).
Byrne, S. et al. Aggregation of neurologic and neuropsychiatric disease in amyotrophic lateral sclerosis kindreds: a population-based case–control cohort study of familial and sporadic amyotrophic lateral sclerosis. Ann. Neurol. 74, 699–708 (2013).
Huisman, M. H. B. et al. Family history of neurodegenerative and vascular diseases in ALS. Neurology 77, 1363-1369 (2011).
Devenney, E. M. et al. Psychiatric disorders in C9orf72 kindreds: study of 1,414 family members. Neurology 91, e1498–e1507 (2018).
O’Brien, M. et al. Clustering of neuropsychiatric disease in first-degree and second-degree relatives of patients with amyotrophic lateral sclerosis. JAMA Neurol. 74, 1425–1430 (2017).
Lin, C.-L. G. et al. Aberrant RNA processing in a neurodegenerative disease: the cause for absent EAAT2, a glutamate transporter, in amyotrophic lateral sclerosis. Neuron 20, 589–602 (1998).
Trotti, D., Rolfs, A., Danbolt, N. C., Brown, R. H. & Hediger, M. A. SOD1 mutants linked to amyotrophic lateral sclerosis selectively inactivate a glial glutamate transporter. Nat. Neurosci. 2, 427–433 (1999).
Neumann, M. et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science 314, 130–133 (2006).
van Eijk, R. P. et al. Meta-analysis of pharmacogenetic interactions in amyotrophic lateral sclerosis clinical trials. Neurology 89, 1915–1922 (2017).
De Schaepdryver, M. et al. Comparison of elevated phosphorylated neurofilament heavy chains in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 89, 367–373 (2018).
Kassubek, J. et al. Imaging the pathoanatomy of amyotrophic lateral sclerosis in vivo: targeting a propagation-based biological marker. J. Neurol. Neurosurg. Psychiatry 89, 374–381 (2018).
Turner, M. R., Kiernan, M. C., Leigh, P. N. & Talbot, K. Biomarkers in amyotrophic lateral sclerosis. Lancet Neurol. 8, 94–109 (2009).
van Eijk, R. P. et al. Monitoring disease progression with plasma creatinine in amyotrophic lateral sclerosis clinical trials. J. Neurol. Neurosurg. Psychiatry 89, 156–161 (2018).
Menon, P. et al. Sensitivity and specificity of threshold tracking transcranial magnetic stimulation for diagnosis of amyotrophic lateral sclerosis: a prospective study. Lancet Neurol. 14, 478–484 (2015).
Geevasinga, N. et al. Riluzole exerts transient modulating effects on cortical and axonal hyperexcitability in ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 17, 580–588 (2016).
Vucic, S. et al. Riluzole exerts central and peripheral modulating effects in amyotrophic lateral sclerosis. Brain 136, 1361–1370 (2013).
Menke, R. A., Agosta, F., Grosskreutz, J., Filippi, M. & Turner, M. R. Neuroimaging endpoints in amyotrophic lateral sclerosis. Neurotherapeutics 14, 11–23 (2017).
McMackin, R. et al. Measuring network disruption in neurodegenerative diseases: new approaches using signal analysis. J. Neurol. Neurosurg. Psychiatry 90, 1011–1020 (2019).
Lu, C.-H. et al. Neurofilament light chain: a prognostic biomarker in amyotrophic lateral sclerosis. Neurology 84, 2247–2257 (2015).
Verde, F. et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 157–164 (2019).
Sormani, M. P. et al. Blood neurofilament light as a potential endpoint in Phase 2 studies in MS. Ann. Clin. Transl. Neurol. 6, 1081–1089 (2019).
Gendron, T. F. et al. Poly (GP) proteins are a useful pharmacodynamic marker for C9ORF72-associated amyotrophic lateral sclerosis. Sci. Transl. Med. 9, eaai7866 (2017).
Mariosa, D. et al. Blood biomarkers of carbohydrate, lipid, and apolipoprotein metabolisms and risk of amyotrophic lateral sclerosis: a more than 20-year follow-up of the Swedish AMORIS cohort. Ann. Neurol. 81, 718–728 (2017).
Takahashi, K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007).
Mertens, J., Marchetto, M. C., Bardy, C. & Gage, F. H. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat. Rev. Neurosci. 17, 424 (2016).
Martinez, A., Del Valle Palomo Ruiz, M., Perez, D. I. & Gil, C. Drugs in clinical development for the treatment of amyotrophic lateral sclerosis. Expert Opin. Investig. Drugs 26, 403–414 (2017).
Yoshida, H. et al. Neuroprotective effects of edaravone: a novel free radical scavenger in cerebrovascular injury. CNS Drug Rev. 12, 9–20 (2006).
Abe, K. et al. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Lunetta, C. et al. The Italian multicenter experience with edaravone in amyotrophic lateral sclerosis. J. Neurol. 267, 3258–3267 (2020).
Al-Chalabi, A. et al. July 2017 ENCALS statement on edaravone. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 471–474 (2017).
Geevasinga, N., Menon, P., Özdinler, P. H., Kiernan, M. C. & Vucic, S. Pathophysiological and diagnostic implications of cortical dysfunction in ALS. Nat. Rev. Neurol. 12, 651 (2016).
Rudzinski, L. A. et al. New antiepileptic drugs: focus on ezogabine, clobazam, and perampanel. J. Investig. Med. 64, 1087–1101 (2016).
Wainger, B. J. et al. Intrinsic membrane hyperexcitability of amyotrophic lateral sclerosis patient-derived motor neurons. Cell Rep. 7, 1–11 (2014).
Wainger, B. J. et al. Effect of ezogabine on cortical and spinal motor neuron excitability in amyotrophic lateral sclerosis. A randomized clinical trial. JAMA Neurol. https://doi.org/10.1001/jamaneurol.2020.4300 (2020).
Sheean, R. K. et al. Association of regulatory T-cell expansion with progression of amyotrophic lateral sclerosis: a study of humans and a transgenic mouse model. JAMA Neurol. 75, 681–689 (2018).
Thonhoff, J. R. et al. Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol. Neuroimmunol. Neuroinflamm. 5, e465 (2018).
Ghadiri, M. et al. Dimethyl fumarate–induced lymphopenia in MS due to differential T-cell subset apoptosis. Neurol. Neuroimmunol. Neuroinflamm. 4, e340 (2017).
Vucic, S. et al. Phase 2 randomized placebo controlled double blind study to assess the efficacy and safety of tecfidera in patients with amyotrophic lateral sclerosis (TEALS Study): Study protocol clinical trial (SPIRIT Compliant). Medicine 99, e18904 (2020).
Burchill, M. A., Yang, J., Vang, K. B. & Farrar, M. A. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 114, 1–8 (2007).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT03039673 (2019).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02059759 (2016).
Camu, W. et al. Repeated 5-day cycles of low dose aldesleukin in amyotrophic lateral sclerosis (IMODALS): a phase 2a randomised, double-blind, placebo-controlled trial. EBioMedicine 59, 102844 (2020).
Mora, J. S. et al. Masitinib as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomized clinical trial. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 5–14 (2020).
Burrage, L. C. et al. Sodium phenylbutyrate decreases plasma branched-chain amino acids in patients with urea cycle disorders. Mol. Genet. Metab. 113, 131–135 (2014).
Obici, L. et al. Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: a phase II study. Amyloid 19, 34–36 (2012).
Cudkowicz, M. E. et al. Phase 2 study of sodium phenylbutyrate in ALS. Amyotroph. Lateral Scler. 10, 99–106 (2009).
Paganoni, S. et al. Trial of sodium phenylbutyrate–taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383, 919–930 (2020).
Paganoni, S. et al. Long-term survival of participants in the CENTAUR trial of sodium phenylbutyrate-taurursodiol in ALS. Muscle Nerve https://doi.org/10.1002/mus.27091 (2020).
TUDCA ALS. New clinical trial for ALS/MND. TUDCA https://www.tudca.eu/ (2020).
Waibel, S., Reuter, A., Malessa, S., Blaugrund, E. & Ludolph, A. C. Rasagiline alone and in combination with riluzole prolongs survival in an ALS mouse model. J. Neurol. 251, 1080–1084 (2004).
Statland, J. M. et al. Rasagiline for amyotrophic lateral sclerosis: a randomized, controlled trial. Muscle Nerve 59, 201–207 (2019).
Ludolph, A. C. et al. Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial. Lancet Neurol. 17, 681–688 (2018).
Turner, M. R. et al. Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol. 12, 310–322 (2013).
Ahmed, R. M. et al. Neuronal network disintegration: common pathways linking neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 87, 1234 (2016).
Eisen, A. et al. Cortical influences drive amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 88, 917 (2017).
Eisen, A., Kiernan, M., Mitsumoto, H. & Swash, M. Amyotrophic lateral sclerosis: a long preclinical period? J. Neurol. Neurosurg. Psychiatry 85, 1232 (2014).
Henderson, R. D., Garton, F. C., Kiernan, M. C., Turner, M. R. & Eisen, A. Human cerebral evolution and the clinical syndrome of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 90, 570–575 (2019).
Kiernan, M. C., Ziemann, U. & Eisen, A. Amyotrophic lateral sclerosis: origins traced to impaired balance between neural excitation and inhibition in the neonatal period. Muscle Nerve 60, 232–235 (2019).
Vucic, S. et al. ALS is a multistep process in South Korean, Japanese, and Australian patients. Neurology 94, e1657 (2020).
Lanka, V., Wieland, S., Barber, J. & Cudkowicz, M. Arimoclomol: a potential therapy under development for ALS. Expert Opin. Investig. Drugs 18, 1907–1918 (2009).
Cudkowicz, M. E. et al. Arimoclomol at dosages up to 300 mg/day is well tolerated and safe in amyotrophic lateral sclerosis. Muscle Nerve 38, 837–844 (2008).
Cha, Y. et al. Drug repurposing from the perspective of pharmaceutical companies. Br. J. Pharmacol. 175, 168–180 (2018).
Cudkowicz, M. E. et al. Safety and efficacy of ceftriaxone for amyotrophic lateral sclerosis: a multi-stage, randomised, double-blind, placebo-controlled trial. Lancet Neurol. 13, 1083–1091 (2014).
Wright, P. D. et al. A high-throughput screen to identify inhibitors of SOD1 transcription. Front. Biosci. 4, 2801-2808 (2012).
Boyd, J. D. et al. A high-content screen identifies novel compounds that inhibit stress-induced TDP-43 cellular aggregation and associated cytotoxicity. J. Biomol. Screen. 19, 44–56 (2014).
Mead, R. J. et al. S [+] Apomorphine is a CNS penetrating activator of the Nrf2-ARE pathway with activity in mouse and patient fibroblast models of amyotrophic lateral sclerosis. Free Radic. Biol. Med. 61, 438–452 (2013).
Benmohamed, R. et al. Identification of compounds protective against G93A-SOD1 toxicity for the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 12, 87–96 (2011).
Oberstadt, M. et al. TDP-43 self-interaction is modulated by redox-active compounds Auranofin, Chelerythrine and Riluzole. Sci. Rep. 8, 2248 (2018).
Nishitoh, H. et al. ALS-linked mutant SOD1 induces ER stress-and ASK1-dependent motor neuron death by targeting Derlin-1. Genes Dev. 22, 1451–1464 (2008).
Groen, E. J., Talbot, K. & Gillingwater, T. H. Advances in therapy for spinal muscular atrophy: promises and challenges. Nat. Rev. Neurol. 14, 214 (2018).
Tan, R. H. et al. TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 138, 3110–3122 (2015).
Miller, T. M. et al. An antisense oligonucleotide against SOD1 delivered intrathecally for patients with SOD1 familial amyotrophic lateral sclerosis: a phase 1, randomised, first-in-man study. Lancet Neurol. 12, 435–442 (2013).
Miller, T. et al. Phase 1–2 trial of antisense oligonucleotide tofersen for SOD1 ALS. N. Engl. J. Med. 383, 109–119 (2020).
Mueller, C. et al. SOD1 suppression with adeno-associated virus and microRNA in familial ALS. N. Engl. J. Med. 383, 151–158 (2020).
Kariyawasam, D., Alexander, I. E., Kurian, M. & Farrar, M. A. Great expectations: virus-mediated gene therapy in neurological disorders. J. Neurol. Neurosurg. Psychiatry 91, 849–860 (2020).
Donnelly, C. J. et al. RNA toxicity from the ALS/FTD C9ORF72 expansion is mitigated by antisense intervention. Neuron 80, 415–428 (2013).
Smith, C. E. & Zain, R. Therapeutic oligonucleotides: state of the art. Annu. Rev. Pharmacol. Toxicol. 59, 605–630 (2019).
Amoasii, L. et al. Gene editing restores dystrophin expression in a canine model of Duchenne muscular dystrophy. Science 362, 86–91 (2018).
Goutman, S. A. et al. Long-term Phase 1/2 intraspinal stem cell transplantation outcomes in ALS. Ann. Clin. Transl. Neurol. 5, 730–740 (2018).
Nicholson, K. A., Cudkowicz, M. E. & Berry, J. D. Clinical trial designs in amyotrophic lateral sclerosis: does one design fit all? Neurotherapeutics 12, 376–383 (2015).
Swash, M. Clinical trials in the ALS syndrome: it is time for change. J. Neurol. Neurosurg. Psychiatry 90, 1308 (2019).
DasMahapatra, P., Raja, P., Gilbert, J. & Wicks, P. Clinical trials from the patient perspective: survey in an online patient community. BMC Health Serv. Res. 17, 166 (2017).
Collet, M. How much does distance limit the pool of potential clinical trial participants in the United States? F1000Research https://doi.org/10.7490/f1000research.1115158.1 (2017).
Cecchini, M. et al. Challenges with novel clinical trial designs: master protocols. Clin. Cancer Res. 25, 2049–2057 (2019).
Hirakawa, A., Asano, J., Sato, H. & Teramukai, S. Master protocol trials in oncology: review and new trial designs. Contemp. Clin. Trials Commun. 12, 1–8 (2018).
Saville, B. R. & Berry, S. M. Efficiencies of platform clinical trials: a vision of the future. Clin. Trials 13, 358–366 (2016).
Connick, P. et al. Multiple sclerosis-secondary progressive multi-arm randomisation trial (MS-SMART): a multiarm phase IIb randomised, double-blind, placebo-controlled clinical trial comparing the efficacy of three neuroprotective drugs in secondary progressive multiple sclerosis. BMJ Open 8, e021944 (2018).
Stern, A. D. & Mehta, S. Adaptive platform trials: the clinical trial of the future? Harvard Business School https://www.hbs.edu/faculty/Pages/item.aspx?num=53315 (2017).
US Food and Drug Administration. Master protocols: efficient clinical trial design strategies to expedite development of oncology drugs and biologics guidance for industry (FDA, 2018).
Rosenfeld, J. Multi-drug therapy in amyotrophic lateral sclerosis: the case for a multi-drug approach. Muscle Nerve 30, 673–675 (2004).
Park, S. B. et al. Flecainide in amyotrophic lateral sclerosis as a neuroprotective strategy (FANS): a randomized placebo-controlled trial. EBioMedicine 2, 1916–1922 (2015).
de Carvalho, M. & Swash, M. Can selection of rapidly progressing patients shorten clinical trials in amyotrophic lateral sclerosis? Arch. Neurol. 63, 557–560 (2006).
Moore, D. H. II & Miller, R. G. Improving efficiency of ALS clinical trials using lead-in designs. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 5 (Suppl. 1), 57–60 (2004).
Al-Chalabi, A. et al. Amyotrophic lateral sclerosis: moving towards a new classification system. Lancet Neurol. 15, 1182–1194 (2016).
Balendra, R. et al. Use of clinical staging in amyotrophic lateral sclerosis for phase 3 clinical trials. J. Neurol. Neurosurg. Psychiatry 86, 45–49 (2015).
Al-Chalabi, A. et al. Oral levosimendan in amyotrophic lateral sclerosis: a phase II multicentre, randomised, double-blind, placebo-controlled trial. J. Neurol. Neurosurg. Psychiatry 90, 1165–1170 (2019).
Smith, R. et al. Enhanced bulbar function in amyotrophic lateral sclerosis: the Nuedexta treatment trial. Neurotherapeutics 14, 762–772 (2017).
Labra, J., Menon, P., Byth, K., Morrison, S. & Vucic, S. Rate of disease progression: a prognostic biomarker in ALS. J. Neurol. Neurosurg. Psychiatry 87, 628–632 (2016).
Gold, J. et al. Safety and tolerability of Triumeq in amyotrophic lateral sclerosis: the Lighthouse trial. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 595–604 (2019).
Atassi, N. et al. The PRO-ACT database: design, initial analyses, and predictive features. Neurology 83, 1719–1725 (2014).
Tramacere, I. et al. The MITOS system predicts long-term survival in amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 86, 1180–1185 (2015).
Fang, T. et al. Stage at which riluzole treatment prolongs survival in patients with amyotrophic lateral sclerosis: a retrospective analysis of data from a dose-ranging study. Lancet Neurol. 17, 416–422 (2018).
Fang, T. et al. Comparison of the King’s and MiToS staging systems for ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. 18, 227–232 (2017).
Iazzolino, B. et al. Validation of the revised classification of cognitive and behavioural impairment in ALS. J. Neurol. Neurosurg. Psychiatry 90, 734 (2019).
Crockford, C. et al. ALS-specific cognitive and behavior changes associated with advancing disease stage in ALS. Neurology 91, e1370–e1380 (2018).
Atassi, N. et al. Analysis of start-up, retention, and adherence in ALS clinical trials. Neurology 81, 1350–1355 (2013).
Kaji, R. et al. Ultra-high-dose methylcobalamin in amyotrophic lateral sclerosis: a long-term phase II/III randomised controlled study. J. Neurol. Neurosurg. Psychiatry 90, 451–457 (2019).
Rutkove, S. B. et al. ALS longitudinal studies with frequent data collection at home: study design and baseline data. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 61–67 (2019).
Wicks, P., Vaughan, T. E., Massagli, M. P. & Heywood, J. Accelerated clinical discovery using self-reported patient data collected online and a patient-matching algorithm. Nat. Biotechnol. 29, 411 (2011).
Rutkove, S. B. Clinical measures of disease progression in amyotrophic lateral sclerosis. Neurotherapeutics 12, 384–393 (2015).
Shefner, J. M. et al. A phase 2, double-blind, randomized, dose-ranging trial of Reldesemtiv in patients with ALS. Amyotroph. Lateral Scler. Frontotemporal Degener. https://doi.org/10.1080/21678421.2020.1822410 (2020).
Maier, A. et al. Online assessment of ALS functional rating scale compares well to in-clinic evaluation: a prospective trial. Amyotroph. Lateral Scler. 13, 210–216 (2012).
Bedlack, R. et al. Lunasin does not slow ALS progression: results of an open-label, single-center, hybrid-virtual 12-month trial. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 285–293 (2019).
ALSUntangled Group. ALSUntangled no. 26: lunasin. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 622–626 (2014).
Bedlack, R. S. et al. How common are ALS plateaus and reversals? Neurology 86, 808–812 (2016).
Paganoni, S. et al. Trial of sodium phenylbutyrate-taurursodiol for amyotrophic lateral sclerosis. N. Engl. J. Med. 383, 919–930 (2020).
Oskarsson, B. et al. Mexiletine for muscle cramps in amyotrophic lateral sclerosis: a randomized, double-blind crossover trial. Muscle Nerve 58, 42–48 (2018).
Weiss, M. D. et al. A randomized trial of mexiletine in ALS: safety and effects on muscle cramps and progression. Neurology 86, 1474–1481 (2016).
Writing Group, Edaravone (MCI-186) ALS Study Group. Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled trial. Lancet Neurol. 16, 505–512 (2017).
Chen, P. C., Hsieh, Y. C., Huang, C. C. & Hu, C. J. Tamoxifen for amyotrophic lateral sclerosis: a randomized double-blind clinical trial. Medicine 99, e20423 (2020).
Babu, S. et al. Selection design phase II trial of high dosages of tamoxifen and creatine in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 21, 15–23 (2020).
Levine, T. D., Bowser, R., Hank, N. & Saperstein, D. A pilot trial of memantine and riluzole in ALS: correlation to CSF biomarkers. Amyotroph. Lateral Scler. 11, 514–519 (2010).
de Carvalho, M. et al. A randomized, placebo-controlled trial of memantine for functional disability in amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. 11, 456–460 (2010).
Macchi, Z. et al. A multi-center screening trial of rasagiline in patients with amyotrophic lateral sclerosis: possible mitochondrial biomarker target engagement. Amyotroph. Lateral Scler. Frontotemporal Degener. 16, 345–352 (2015).
Ludolph, A. C. et al. Safety and efficacy of rasagiline as an add-on therapy to riluzole in patients with amyotrophic lateral sclerosis: a randomised, double-blind, parallel-group, placebo-controlled, phase 2 trial. Lancet Neurol. 17, 681–688 (2018).
Benatar, M. et al. Randomized, double-blind, placebo-controlled trial of arimoclomol in rapidly progressive SOD1 ALS. Neurology 90, e565–e574 (2018).
Acknowledgements
This manuscript was prepared by members of ForeFront, a large collaborative research group dedicated to the study of neurodegenerative diseases and funded by the National Health and Medical Research Council of Australia Program Grant (#1132524), a Dementia Research Team Grant (#1095127) and a Partnership Project (1153439). M.C.K. is supported by an NHMRC Practitioner Fellowship (1156093). J.M.S. receives funding from ALS Finding a Cure Foundation. A.A.-C. is supported through the United Kingdom Medical Research Council (MR/R024804/1) under the aegis of JPND (www.jpnd.eu), the Motor Neurone Disease Association, and the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. P.W. is employed by Wicks Digital Health, which has received funding from Ada Health, AstraZeneca, Baillie Gifford, Bold Health, Camoni, Compass Pathways, Coronna, EIT, Happify, HealthUnlocked, Inbeeo, Kheiron Medical, Sano Genetics, Self Care Catalysts, The Learning Corp, The Wellcome Trust, VeraSci and Woebot. M.R.T. is supported by the Motor Neurone Disease Association.
Author information
Authors and Affiliations
Contributions
M.C.K. researched data for the article. M.C.K. and S.V. made substantial contributions to discussion of the content. M.C.K., S.V., K.T., C.J.M., O.H., J.M.S., A.A.-C. and M.R.T. contributed to the writing of the article. All authors reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Neurology thanks M. de Carvalho, C. Lunetta, S. Petri and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ALS Therapy Development Institute: www.als.net
ANSWER ALS: www.answerals.org
PatientsLikeMe: www.patientslikeme.com
TARGET ALS: www.targetals.org
Rights and permissions
About this article
Cite this article
Kiernan, M.C., Vucic, S., Talbot, K. et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat Rev Neurol 17, 104–118 (2021). https://doi.org/10.1038/s41582-020-00434-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41582-020-00434-z
This article is cited by
-
Gene therapy breakthroughs in ALS: a beacon of hope for 20% of ALS patients
Translational Neurodegeneration (2025)
-
Targeting common disease pathomechanisms to treat amyotrophic lateral sclerosis
Nature Reviews Neurology (2025)
-
High-density multielectrode arrays bring cellular resolution to neuronal activity and network analyses of corticospinal motor neurons
Scientific Reports (2025)
-
Longitudinal assessment of cortical motor function in amyotrophic lateral sclerosis
Scientific Reports (2025)
-
Cognitive impairment within and beyond the FTD spectrum in ALS: development of a complementary cognitive screen
Journal of Neurology (2025)