Abstract
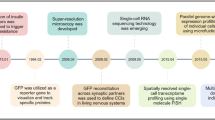
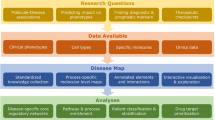
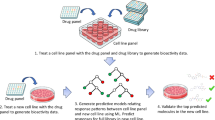
Here we describe the LifeTime Initiative, which aims to track, understand and target human cells during the onset and progression of complex diseases, and to analyse their response to therapy at single-cell resolution. This mission will be implemented through the development, integration and application of single-cell multi-omics and imaging, artificial intelligence and patient-derived experimental disease models during the progression from health to disease. The analysis of large molecular and clinical datasets will identify molecular mechanisms, create predictive computational models of disease progression, and reveal new drug targets and therapies. The timely detection and interception of disease embedded in an ethical and patient-centred vision will be achieved through interactions across academia, hospitals, patient associations, health data management systems and industry. The application of this strategy to key medical challenges in cancer, neurological and neuropsychiatric disorders, and infectious, chronic inflammatory and cardiovascular diseases at the single-cell level will usher in cell-based interceptive medicine in Europe over the next decade.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
27,99 € / 30 days
cancel any time
Subscribe to this journal
Receive 51 print issues and online access
199,00 € per year
only 3,90 € per issue
Buy this article
- Purchase on SpringerLink
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Change history
17 March 2021
A Correction to this paper has been published: https://doi.org/10.1038/s41586-021-03287-8
References
Claussnitzer, M. et al. A brief history of human disease genetics. Nature 577, 179–189 (2020).
Karczewski, K. J. & Snyder, M. P. Integrative omics for health and disease. Nat. Rev. Genet. 19, 299–310 (2018).
Clevers, H. Modeling development and disease with organoids. Cell 165, 1586–1597 (2016).
Lancaster, M. A. & Knoblich, J. A. Organogenesis in a dish: modeling development and disease using organoid technologies. Science 345, 1247125 (2014).
Tanay, A. & Regev, A. Scaling single-cell genomics from phenomenology to mechanism. Nature 541, 331–338 (2017).
Regev, A. et al. The human cell atlas. eLife 6, e27041 (2017).
The LifeTime Initiative. LifeTime Strategic Research Agenda. https://lifetime-initiative.eu/wp-content/uploads/2020/08/LifeTime-Strategic-Research-Agenda.pdf (2020).
Yofe, I., Dahan, R. & Amit, I. Single-cell genomic approaches for developing the next generation of immunotherapies. Nat. Med. 26, 171–177 (2020).
HuBMAP Consortium. The human body at cellular resolution: the NIH Human Biomolecular Atlas Program. Nature 574, 187–192 (2019).
Guo, X. et al. Global characterization of T cells in non-small-cell lung cancer by single-cell sequencing. Nat. Med. 24, 978–985 (2018).
Ledergor, G. et al. Single cell dissection of plasma cell heterogeneity in symptomatic and asymptomatic myeloma. Nat. Med. 24, 1867–1876 (2018).
Li, H. et al. Dysfunctional CD8 T cells form a proliferative, dynamically regulated compartment within human melanoma. Cell 176, 775–789.e718 (2019).
Puram, S. V. et al. Single-cell transcriptomic analysis of primary and metastatic tumor ecosystems in head and neck cancer. Cell 171, 1611–1624.e1624 (2017).
Tirosh, I. et al. Dissecting the multicellular ecosystem of metastatic melanoma by single-cell RNA-seq. Science 352, 189–196 (2016).
van Galen, P. et al. Single-cell RNA-seq reveals AML hierarchies relevant to disease progression and immunity. Cell 176, 1265–1281.e1224 (2019).
Der, E. et al. Tubular cell and keratinocyte single-cell transcriptomics applied to lupus nephritis reveal type I IFN and fibrosis relevant pathways. Nat. Immunol. 20, 915–927 (2019).
Zhang, F. et al. Defining inflammatory cell states in rheumatoid arthritis joint synovial tissues by integrating single-cell transcriptomics and mass cytometry. Nat. Immunol. 20, 928–942 (2019).
Grubman, A. et al. A single-cell atlas of entorhinal cortex from individuals with Alzheimer’s disease reveals cell-type-specific gene expression regulation. Nat. Neurosci. 22, 2087–2097 (2019).
Keren-Shaul, H. et al. A unique microglia type associated with restricting development of Alzheimer’s disease. Cell 169, 1276–1290.e1217 (2017).
Mathys, H. et al. Single-cell transcriptomic analysis of Alzheimer’s disease. Nature 570, 332–337 (2019).
Wang, L. et al. Single-cell reconstruction of the adult human heart during heart failure and recovery reveals the cellular landscape underlying cardiac function. Nat. Cell Biol. 22, 108–119 (2020).
Reyes, M. et al. An immune-cell signature of bacterial sepsis. Nat. Med. 26, 333–340 (2020).
Argelaguet, R. et al. Multi-omics profiling of mouse gastrulation at single-cell resolution. Nature 576, 487–491 (2019).
Clark, S. J. et al. scNMT-seq enables joint profiling of chromatin accessibility DNA methylation and transcription in single cells. Nat. Commun. 9, 781 (2018).
Rooijers, K. et al. Simultaneous quantification of protein–DNA contacts and transcriptomes in single cells. Nat. Biotechnol. 37, 766–772 (2019).
Chen, W. T. et al. Spatial transcriptomics and in situ sequencing to study Alzheimer’s disease. Cell 182, 976–991.e19 (2020).
Giladi, A. et al. Dissecting cellular crosstalk by sequencing physically interacting cells. Nat. Biotechnol. 38, 629–637 (2020).
Moffitt, J. R. et al. Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324 (2018).
Nitzan, M., Karaiskos, N., Friedman, N. & Rajewsky, N. Gene expression cartography. Nature 576, 132–137 (2019).
Ståhl, P. L. et al. Visualization and analysis of gene expression in tissue sections by spatial transcriptomics. Science 353, 78–82 (2016).
van den Brink, S. C. et al. Single-cell and spatial transcriptomics reveal somitogenesis in gastruloids. Nature 582, 405–409 (2020).
Vickovic, S. et al. High-definition spatial transcriptomics for in situ tissue profiling. Nat. Methods 16, 987–990 (2019).
Bintu, B. et al. Super-resolution chromatin tracing reveals domains and cooperative interactions in single cells. Science 362, eaau1783 (2018).
Cardozo Gizzi, A. M. et al. Microscopy-based chromosome conformation capture enables simultaneous visualization of genome organization and transcription in intact organisms. Mol. Cell. 74, 212–222.e215 (2019).
Chen, K. H., Boettiger, A. N., Moffitt, J. R., Wang, S. & Zhuang, X. RNA imaging. Spatially resolved, highly multiplexed RNA profiling in single cells. Science 348, aaa6090 (2015).
Mateo, L. J. et al. Visualizing DNA folding and RNA in embryos at single-cell resolution. Nature 568, 49–54 (2019).
Medaglia, C. et al. Spatial reconstruction of immune niches by combining photoactivatable reporters and scRNA-seq. Science 358, 1622–1626 (2017).
Jackson, H. W. et al. The single-cell pathology landscape of breast cancer. Nature 578, 615–620 (2020).
Keren, L. et al. A structured tumor-immune microenvironment in triple negative breast cancer revealed by multiplexed ion beam imaging. Cell 174, 1373–1387.e1319 (2018).
Maniatis, S. et al. Spatiotemporal dynamics of molecular pathology in amyotrophic lateral sclerosis. Science 364, 89–93 (2019).
Baron, C. S. & van Oudenaarden, A. Unravelling cellular relationships during development and regeneration using genetic lineage tracing. Nat. Rev. Mol. Cell Biol. 20, 753–765 (2019).
Helmink, B. A. et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 577, 549–555 (2020).
Krieg, C. et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat. Med. 24, 144–153 (2018).
Kim, C. et al. Chemoresistance evolution in triple-negative breast cancer delineated by single-cell sequencing. Cell 173, 879–893.e813 (2018).
Rambow, F. et al. Toward minimal residual disease-directed therapy in melanoma. Cell 174, 843–855.e819 (2018).
Corcoran, R. B. & Chabner, B. A. Application of cell-free DNA analysis to cancer treatment. N. Engl. J. Med. 379, 1754–1765 (2018).
Eraslan, G., Avsec, Ž., Gagneur, J. & Theis, F. J. Deep learning: new computational modelling techniques for genomics. Nat. Rev. Genet. 20, 389–403 (2019).
Lähnemann, D. et al. Eleven grand challenges in single-cell data science. Genome Biol. 21, 31 (2020).
Topol, E. J. High-performance medicine: the convergence of human and artificial intelligence. Nat. Med. 25, 44–56 (2019).
Argelaguet, R. et al. Multi-Omics Factor Analysis—a framework for unsupervised integration of multi-omics data sets. Mol. Syst. Biol. 14, e8124 (2018).
Efremova, M. & Teichmann, S. A. Computational methods for single-cell omics across modalities. Nat. Methods 17, 14–17 (2020).
Pearl, J. & Mackenzie, D. The Book of Why: The New Science of Cause and Effect (Penguin, 2019).
Amin, N. D. & Paşca, S. P. Building models of brain disorders with three-dimensional organoids. Neuron 100, 389–405 (2018).
Knoblich, J. A. Lab-built brains. Sci. Am. 316, 26–31 (2016).
Bleijs, M., van de Wetering, M., Clevers, H. & Drost, J. Xenograft and organoid model systems in cancer research. EMBO J. 38, e101654 (2019).
Byrne, A. T. et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat. Rev. Cancer 17, 254–268 (2017).
Espuny-Camacho, I. et al. Hallmarks of Alzheimer’s disease in stem-cell-derived human neurons transplanted into mouse brain. Neuron 93, 1066–1081.e1068 (2017).
Hasselmann, J. et al. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron 103, 1016–1033.e1010 (2019).
Mancuso, R. et al. Stem-cell-derived human microglia transplanted in mouse brain to study human disease. Nat. Neurosci. 22, 2111–2116 (2019).
Wilkinson, M. D. et al. The FAIR guiding principles for scientific data management and stewardship. Sci. Data 3, 160018 (2016).
Schultze, J. L.The SYSCID Consortium & Rosenstiel, P. Systems medicine in chronic inflammatory diseases. Immunity 48, 608–613 (2018).
Life Science RI European Life Science Research Infrastructures https://lifescience-ri.eu/home.html (2020).
Sugarman, J. & Bredenoord, A. L. Real-time ethics engagement in biomedical research: ethics from bench to bedside. EMBO Rep. 21, e49919 (2020).
Torres-Padilla, M. E. et al. Thinking ‘ethical’ when designing a new biomedical research consortium. EMBO J. 39, e105725 (2020).
European Commission. People in the EU: who are we and how do we live? https://ec.europa.eu/eurostat/documents/3217494/7089681/KS-04-15-567-EN-N.pdf/8b2459fe-0e4e-4bb7-bca7-7522999c3bfd (Eurostat, 2015).
What happened to personalized medicine? Nat. Biotechnol. 30, 1 (2012).
Acknowledgements
We acknowledge all participants that have attended and contributed to LifeTime meetings and workshops through many presentations and discussions. We thank J. Richers for artwork and A. Sonsala, A. Tschernycheff and C. Lozach for administrative support. LifeTime has received funding from the European Union’s Horizon 2020 research and innovation framework programme under grant agreement 820431.
Author information
Authors and Affiliations
Consortia
Contributions
All authors contributed to the writing of the article and provided comments and feedback. They all approved submission of the article for publication. The individuals listed at the end of the paper are members of Working Groups that contributed to the writing of the LifeTime Strategic Research Agenda (listed in full in the Supplementary Information). Please note that the complete LifeTime Community is much broader and includes many associates and supporters that are actively contributing to and advocating for LifeTime (further information can be found at https://lifetime-initiative.eu).
Corresponding authors
Ethics declarations
Competing interests
C.B. is an inventor on several patent applications in genome technology and cofounder of Aelian Biotechnology, a single-cell CRISPR screening company. H.C. is a non-executive board member of Roche Holding, Basel. A.P. holds European and US patents on ‘Genome Architecture Mapping’ (EP 3230465 B1, US 10526639 B2). W.R. is a consultant and shareholder of Cambridge Epigenetix. T.V. is co-inventor on licensed patents WO/2011/157846 (methods for haplotyping single cells), WO/2014/053664 (high-throughput genotyping by sequencing low amounts of genetic material), WO/2015/028576 (haplotyping and copy number typing using polymorphic variant allelic frequencies). All other authors declare no competing interests.
Additional information
Peer review information Nature thanks Michael Snyder, Ali Torkamani and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information
Author and affiliation list for members of the LifeTime Working Groups.
Rights and permissions
About this article
Cite this article
Rajewsky, N., Almouzni, G., Gorski, S.A. et al. LifeTime and improving European healthcare through cell-based interceptive medicine. Nature 587, 377–386 (2020). https://doi.org/10.1038/s41586-020-2715-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41586-020-2715-9
This article is cited by
-
Multiplexing cortical brain organoids for the longitudinal dissection of developmental traits at single-cell resolution
Nature Methods (2025)
-
The 37TrillionCells initiative for improving global healthcare via cell-based interception and precision medicine: focus on neurodegenerative diseases
Molecular Brain (2024)
-
StaVia: spatially and temporally aware cartography with higher-order random walks for cell atlases
Genome Biology (2024)
-
Panpipes: a pipeline for multiomic single-cell and spatial transcriptomic data analysis
Genome Biology (2024)
-
Genetics of human brain development
Nature Reviews Genetics (2024)